A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Live Imaging of Early Cardiac Progenitors in the Mouse Embryo
In This Article
Summary
We present a detailed protocol for mouse embryo culture and imaging that enables 3D + time imaging of cardiac progenitor cells. This video-toolkit addresses the key skills required for successful live imaging otherwise hard to acquire from text-only publications.
Abstract
The first steps of heart development imply drastic changes in cell behavior and differentiation. While analysis of fixed embryos allows studying in detail specific developmental stages in a still snapshot, live imaging captures dynamic morphogenetic events, such as cell migration, shape changes, and differentiation, by imaging the embryo as it develops. This complements fixed analysis and expands the understanding of how organs develop during embryogenesis. Despite its advantages, live imaging is rarely used in mouse models because of its technical challenges. Early mouse embryos are sensitive when cultured ex vivo and require efficient handling. To facilitate a broader use of live imaging in mouse developmental research, this paper presents a detailed protocol for two-photon live microscopy that allows long-term acquisition in mouse embryos. In addition to the protocol, tips are provided on embryo handling and culture optimization. This will help understand key events in early mouse organogenesis, enhancing the understanding of cardiovascular progenitor biology.
Introduction
The heart forms early during embryogenesis to start pumping nutrients to the whole embryo, while it continues developing1. In mouse embryos, one and a half days after the initiation of gastrulation, a rudimentary heart organ assembles at the anterior pole2,3. By Early Streak (ES) stage, cardiac progenitors in the epiblast ingress through the primitive streak to the nascent mesodermal layer4,5,6 and start migrating to the anterior pole, where they differentiate to form the primitive heart tube. Throughout this process, early heart progenitors undergo cell rearrangements, shape transformations, and differentiation, in addition to migration7 (Figure 1).
Early cardiac progenitors have attracted researchers for nearly a century due to their remarkable ability to differentiate and build a functional organ simultaneously. Over the last two decades, clonal analysis and conditional knockout models have shown that early heart development implicates distinct cell sources in a highly dynamic process8,9,10. However, the 3D structure of the primitive heart tube and the dynamic nature of its morphogenesis make it challenging to study (Figure 1), and we are far from understanding its full complexity11.
To study these dynamic cellular processes, live imaging methods now offer an unprecedented detail7,12,13,14. In the mouse model, live approaches have been key to interrogating developmental topics that are difficult to address by static analysis7,13,15. While long-term ex vivo culture and robust microscope setups are advancing fast16,17, few researchers have the expertise to successfully image live embryos. Although paper-based publications provide enough technical details to reproduce live imaging experiments, some skills and tricks are hard to grasp without visual examples or peer-to-peer assistance. To accelerate this learning process and spread the use of live imaging among laboratories, we assembled a video protocol (Figure 2) that gathers the necessary skills to perform live imaging on gastrulating mouse embryos.
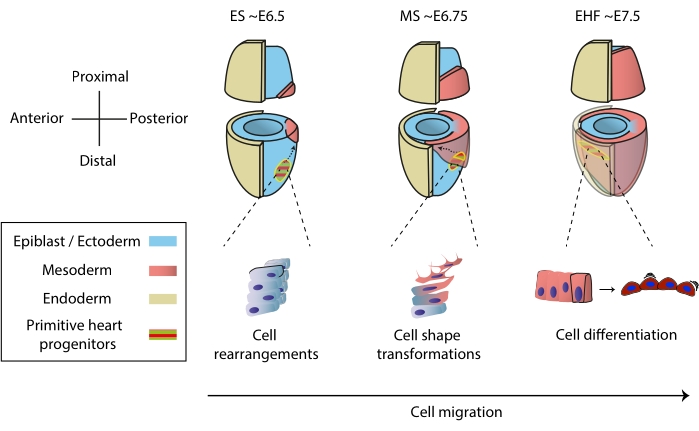
Figure 1: Early differentiation of cardiac progenitor cells in the mouse embryo from the onset of gastrulation to the stage preceding primitive heart tube formation. Cardiac progenitor cells ingress the mesoderm soon after the start of gastrulation, migrating to the opposite side of the embryo. Morphological and embryonic day (E) stage are written on top of the diagrams. Dashed arrows depict the migration trajectory of primitive heart tube progenitors during gastrulation. This figure was adapted from11. Abbreviations: ES = Early Streak; MS = Middle Streak; EHF = Early Head Fold. Please click here to view a larger version of this figure.

Figure 2: Workflow diagram for live imaging of early heart progenitors. Please click here to view a larger version of this figure.
Access restricted. Please log in or start a trial to view this content.
Protocol
All animal procedures were approved by the CNIC Animal Experimentation Ethics Committee, by the Community of Madrid (Reference PROEX 220/15) and conformed to EU Directive 2010/63EU and Recommendation 2007/526/EC regarding the protection of animals used for experimental and other scientific purposes, enforced in Spanish law under Real Decreto 1201/2005.
This protocol includes the use of two males from the fluorescent transgenic mouse line reporting NOTCH activity Tg(CBF:H2BVenus,+)18. See the Table of Materials for details about materials, animals, and equipment used in this protocol.
1. Tools and holder customization
- Cut 1 cm long tungsten wire pieces and sharpen them to 0.02-0.05 mm diameter using any of the simple methods available, for example, submerging the tip of the wire in a saturated sodium nitrite solution19.
- Prepare elbowed glass capillaries.
- Place the midpoint of a standard 1.0 mm glass capillary over a Bunsen lighter for 3-6 s, pulling slowly from both ends until the capillary turns thin and flexible. At that point, break the capillary in half and heat it briefly to produce a 90° bend 2 cm from the tip.
- Saw a piece of polymethyl methacrylate measuring approximately 7 mm long, 4 mm wide, and 2 mm thick (Figure 3D).
NOTE: Polymethyl methacrylate sheets can be either obtained from recycled laboratory equipment or ordered new. - Hold the methacrylate piece using a bench vise. Next, drill custom-sized holes transversally through the whole piece (Figure 3D).
NOTE: Typically, E6.5 to E7.5 mouse embryos require using 0.2-0.5 mm diameter drills.- Rotate the drill slowly while applying low but constant pressure. If a drill breaks inside the holder, discard the piece, as the metal cannot be taken out.
- Use a fine file to smooth the holder's edges and adjust its size. Additionally, create a mild slope on the edges of the holder to facilitate embryo positioning (Figure 3D).
- Place the finished holder in a dish with distilled water to rinse it.
- Check the holder under the stereomicroscope to see if the holes contain drilling dust. If dust is present, use tungsten needles to remove it.
- To sterilize the holder, place it in a conical tube full of distilled water and sonicate it for 20 min with a minimum 20 W power output.
2. Media preparation
- To heat-inactivate the complement system, leave two 500 μL aliquots of commercial or homemade20 rat serum at 56 °C for 30 min.
NOTE: For a detailed protocol on how to prepare rat serum, see21. - In a cell culture hood, prepare two aliquots of the medium for culturing the embryos in the microscope. For each, mix 490 μL of DMEM for live-cell imaging, 500 μL of inactivated rat serum, and 10 μL of penicillin-streptomycin to obtain a final concentration of 50 µg/mL penicillin and 50 µg/mL streptomycin22.
NOTE: The volume of culture medium depends on the time and number of embryos to be cultured. As a rule of thumb, optimal growth requires a minimum of 200 μL per embryo per day in E7.0 embryos. - Using a 2 mL syringe, filter the medium through a 0.22 µm pore size membrane filter into a new tube and place it lid-open in a cell culture incubator at 37 °C and 7% CO2 for 1 h before embryo culture23.
- Prepare the dissection medium by adding to a 500 mL of DMEM supplemented with L-glutamine bottle the following: 50 mL of fetal bovine serum, 10 mL of 25 mM HEPES-NaOH (pH 7.2), and 10 mL of penicillin and streptomycin (50 mg/mL).
- Next, separate it into 50 mL aliquots, place three aliquots in a 37 °C bath and store the rest at 4 °C for up to 3 months.
3. Embryo dissection
- Euthanize an E6.75-E7.5 pregnant mouse.
- Extract the uterus and place it on a dry and clean paper wipe. Then, cut the uterus, inserting the tip of fine scissors from the mesometrial side and sliding the blade along to expose the deciduae and transfer them to the dissection medium. For a detailed protocol, see24.
- Dissect the embryos, keeping the ectoplacental cone intact (Figure 3A,B). For a detailed dissection method, refer to25.
- Then, peel off the Reichert's membrane, leaving part of it on the extraembryonic region (Figure 3B).
NOTE: Fine forceps are essential for this step. Inexperienced users can dissect embryos on 2% agarose gel-coated dishes to avoid bending the forceps' tips against the dish surface (Figure 3C). - To keep the dissection medium warm, replace it every 10 min. Moreover, dissect deciduae in groups of 3-4 while keeping the rest in dissection medium placed in a 37 °C bath. Immediately place the dissected embryos in culture medium.
- Select the embryos with the desired fluorescence features using a fluorescence stereomicroscope and place them in the tube containing culture medium in the cell culture incubator.
- If the embryos are at E6.5 to E7.0 stages, close the lid and leave the embryos to recover for 2 h before imaging.
NOTE: E6.5 to E7.0 embryos are sensitive. They can also be placed directly under the microscope but preculturing allows for selection of the ones that best recover from dissection, increasing the chance of success.
4. Microscope preparation
- Turn on all microscope components, including lasers, computer, and software. Turn on the temperature controllers and set to 37 °C 3 h in advance to ensure equilibration.
- If using an immersion detection objective, clean it using a residue-free disposable wipe. Dip the objective's tip in double-distilled water using a 60 mm dish and leave it until the acquisition starts.
- Turn on the CO2 controller and set it to 7%.
- Place a drop of high-vacuum silicon grease on a 35 mm dish with glass coverslip bottom (14 mm diameter), place the holder on top of the drop, and press gently to immobilize it.
- Under a stereomicroscope, add culture medium to fill the glass-bottom portion of the dish. While doing so, aim the stream to the holder's holes to prevent formation of air bubbles in them.
- If the bubbles remain, use the glass capillary prepared in step 1.2, connected to a silicone tube with a mouthpiece, to suck the bubbles out gently.
NOTE: The elbowed end of the capillary will facilitate access through the holes.
5. Embryo mounting
- Transfer 2-3 embryos that were left to recover in the incubator to the dish with the holder. Leave the rest in the incubator as a backup.
- Set the embryos in the holes by using a pair of forceps and a tapered tungsten needle. Use the needle to hook the embryo by the ectoplacental cone and insert it into a size-matching hole. To immobilize the embryo, rotate the cone by 90-120° so that the Reichter's membrane adheres to the hole walls (Figure 3C). For an alternative description, see26.
- Carefully move the dish containing the embryos and the holder to the microscope plate (Figure 3F,G).
NOTE: If not moved steadily, the embryo can move out of the holder. - Lower the objective until a liquid meniscus is formed at the medium-objective interface.
- Locate the embryo using transmitted light under the microscope binocular and place the embryo in focus.
- Mount the incubation chamber and seal it around the objective using tape (Figure 3F).
- Using a Live Capture at the microscope control software, adjust the Laser Output and Gain levels.
NOTE: For two-photon acquisition of green fluorescent protein (GFP) and Tdtomato reporters, 30% output laser power at 980 nm and 70% gain on non-descanned detectors were used in this protocol (Figure 3G). - To avoid evaporation, cover the medium with paraffin oil by dripping it slowly on the objective.
NOTE: This is the last point where additional embryos can be mounted in the holder. Once covered with paraffin oil, one would have to clean the holder and replace the medium to do so. For alternative mounting methods see27.
6. Image acquisition
- Set up time-lapse recording. Set a 5-10 min time interval with 3-5 µm z-space between stacks. Leave a blank space on top of the z-stack to anticipate embryo growth, a minimum of 50 µm, adding 30 µm for every hour the acquisition will not be supervised.
- If available, enable the Auto-Save option to allow supervision of the acquisition from a personal computer to allow the adjustment of the z-stack size, if needed, to fit the area of interest within focus.

Figure 3: Live imaging tools and setup. (A) Dissecting mouse embryos with an agarose-coated dish under the stereomicroscope. (B) Diagram of the steps to dissect mouse embryos for live imaging. (C) Embryo positioning in the holder. (D) Embryo holder design and features. (F) Incubator chamber around the immersion objective. (G) Diagram of final setup for time-lapse acquisition. Scale bar = 500 µm (A). Please click here to view a larger version of this figure.
Access restricted. Please log in or start a trial to view this content.
Results
We used the protocol to visualize NOTCH signaling activation in early cardiac progenitors about to differentiate to endothelial cells during primitive heart tube morphogenesis. For that, we crossed wild-type C57BL/6-N mice with Tg(CBF:H2BVenus,+) mice18 to obtain embryos reporting NOTCH activity through yellow fluorescent protein Venus. At E7.5, Venus fluorescence is present throughout the neural ectoderm, with a few positive nuclei at the splanchnic and extraembryonic mesoderm. After 4 h, endothe...
Access restricted. Please log in or start a trial to view this content.
Discussion
Early heart progenitors organize in a primitive heart tube that starts beating while it is still forming. Understanding how this process takes place is key to pinpoint the wide spectrum of congenital heart defects to specific morphogenetic events. For that, live imaging offers an opportunity to study normal and defective embryonic development with increased temporal resolution. This is especially useful to study early cardiac progenitor cells as they transition quickly through multiple differentiation and migration behav...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors acknowledge Dr. Kenzo Ivanovitch for previous work on this method and the group of Dr. Shigenori Nonaka (National Institutes of Natural Sciences, Japan) for providing the initial expertise on embryo mounting. This study was supported by Grant PGC2018-096486-B-I00 from the Spanish Ministerio de Ciencia e Innovación and Grant H2020-MSCA-ITN-2016-722427 from the EU Horizon 2020 program to MT and Grant 1380918 from the FEDER Andalucía 2014-2020 Operating Program to JND. MS was supported by a La Caixa Foundation PhD fellowship (LCF/BQ/DE18/11670014) and The Company of Biologists travelling fellowship (DEVTF181145). The CNIC is supported by the Spanish Ministry of Science and the ProCNIC Foundation.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| #55 Forceps | Dumont | 11295-51 | |
| 35 mm Dish with glass coverslip bottom 14 mm Diameter | Mattek | P35G-1.5-14-C | |
| 35 mm vise table | Grandado | SKU 8798771617573 | |
| 50 mL tubes | BD Falcon | 352070 | |
| Distilled water | |||
| DMEM - Dulbecco's Modified Eagle Medium | Gibco | 11966025 | with L-Glutamine, without Glucose, without Na Pyruvate |
| Fetal Bovine Serum | Invitrogen | 10438-026 | |
| Fluorescent reporter transgenic mice (Tg(CBF:H2BVenus,+) | JAX | ||
| Fluorobrite DMEM | ThermoFisher | A1896701 | DMEM for live-cell imaging |
| High-vacuum silicone grease | Dow Corning | Z273554-1EA | |
| Holder for wires | Perlen Pressen | pwb1 | |
| LSM 780 Upright microscope | Zeiss | ||
| MaiTai Deepsee far red pulsed-laser tuned at 980 nm | Spectra-Physics | ||
| Non Descanned Detectors equipped with the filter sets cyan-yellow (BP450-500/BP520-560), green-red (BP500-520/BP570-610) and yellow-red (BP520-560/BP645-710) | Zeiss | ||
| Obj: 20x water dipping 1.0 NA, long working distance | Zeiss | ||
| P1000 and P200 pipettes | |||
| Paraffin Oil | Nidacon | VNI0049 | |
| Penicillin-streptomycin | Invitrogen | 15070-063 | (the final concentration should be 50 μg/mL penicillin and 50 μg/mL streptomycin) |
| Petri dishes 35 mm x 10 mm | BD Falcon | 351008 | |
| Pipette tips | |||
| Polymethyl methacrylate | Reused from old laboratory equipment | ||
| Rat Serum culture embryo, male rats SPRAGUE DAWLEY RjHan SD | Janvier Labs | 9979 | |
| Set of 160 mm fines | RS PRO | 541-6933 | |
| Standard 1.0 mm glass capillaries | Anima Lab | 1B100F-3 | |
| Sterile 0.22 μm syringe filter | Corning | 431218 | |
| Sterile 5 mL syringe | Fisher Scientific | 15809152 | |
| Tungsten needles | |||
| Ultrasonic homogeniser (sonicator) | Bandelin | BASO_17021 |
References
- Tyser, R. C. V., et al. Calcium handling precedes cardiac differentiation to initiate the first heartbeat. eLife. 5, 17113(2016).
- Kelly, R. G., Buckingham, M. E., Moorman, A. F. Heart fields and cardiac morphogenesis. Cold Spring Harbor Perspectives in Medicine. 4 (10), 015750(2014).
- Evans, S. M., Yelon, D., Conlon, F. L., Kirby, M. L. Myocardial lineage development. Circulation Research. 107 (12), 1428-1444 (2010).
- Tam, P. P., Parameswaran, M., Kinder, S. J., Weinberger, R. P. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development. 124 (9), Cambridge, England. 1631-1642 (1997).
- Kinder, S. J., Loebel, D. A. F., Tam, P. P. L. Allocation and early differentiation of cardiovascular progenitors in the mouse embryo. Trends in Cardiovascular Medicine. 11 (5), 177-184 (2001).
- Lawson, K. A. Fate mapping the mouse embryo. International Journal of Developmental Biology. 43 (7), 773-775 (1999).
- Ivanovitch, K., Temiño, S., Torres, M. Live imaging of heart tube development in mouse reveals alternating phases of cardiac differentiation and morphogenesis. eLife. 6, 30668(2017).
- Meilhac, S. M., Buckingham, M. E. The deployment of cell lineages that form the mammalian heart. Nature Reviews Cardiology. 15 (11), 705-724 (2018).
- Buckingham, M., Meilhac, S., Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nature Reviews Genetics. 6 (11), 826-835 (2005).
- Meilhac, S. M., Lescroart, F., Blanpain, C. D., Buckingham, M. E. Cardiac cell lineages that form the heart. Cold Spring Harbor Perspectives in Medicine. 4 (9), 013888(2014).
- Sendra, M., Domínguez, J. N., Torres, M., Ocaña, O. H. Dissecting the complexity of early heart progenitor cells. Journal of Cardiovascular Development and Disease. 9 (1), 5(2022).
- McDole, K., et al. In toto imaging and reconstruction of post-implantation mouse development at the single-cell level. Cell. 175 (3), 859-876 (2018).
- Saykali, B., et al. Distinct mesoderm migration phenotypes in extra-embryonic and embryonic regions of the early mouse embryo. eLife. 8, 42434(2019).
- Ichikawa, T., et al. Live imaging of whole mouse embryos during gastrulation: Migration analyses of epiblast and mesodermal cells. PLoS ONE. 8 (7), 64506(2013).
- Tyser, R. C. V., et al. Single-cell transcriptomic characterization of a gastrulating human embryo. Nature. 600 (7888), 285-289 (2021).
- Aguilera-Castrejon, A., et al. Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis. Nature. 593 (7857), 119-124 (2021).
- Yue, Y., et al. in toto live imaging of cardiomyocyte behaviour during mouse ventricle chamber formation at single-cell resolution. Nature Cell Biology. 22 (3), 332-340 (2020).
- Nowotschin, S., Xenopoulos, P., Schrode, N., Hadjantonakis, A. K. A bright single-cell resolution live imaging reporter of Notch signaling in the mouse. BMC Developmental Biology. 13 (1), 15(2013).
- Cold Spring Harbor Protocols. Sharpened tungsten needles. Cold Spring Harbor Protocols. , (2012).
- Tam, P. P., Snow, M. H. The in vitro culture of primitive-streak-stage mouse embryos. Journal of Embryology and Experimental Morphology. 59, 131-143 (1980).
- Garcia, M. D., Udan, R. S., Hadjantonakis, A. K., Dickinson, M. E. Preparation of rat serum for culturing mouse embryos. Cold Spring Harbor Protocols. 2011 (4), 5593(2011).
- Tam, P. P. L. Postimplantation mouse development: Whole embryo culture and micro- manipulation. International Journal of Developmental Biology. 42 (7), 895-902 (1998).
- González Hinojosa, F. Optimización de propiedades fisicoquímicas y medios de cultivo para el cultivo del embrión de ratón ex vivo. Universidad de Jaén. Biología Experimental. , Available from: https://hdl.handle.net/10953.1/1400 (2021).
- Behringer, R., Gertsenstein, M., Vintersen Nagy, K., Nagy, A. Manipulating the mouse embryo: A laboratory manual, Fourth Edition. , Cold Harbor Laboratory Press. 814(2014).
- Shea, K., Geijsen, N. Dissection of 6.5 dpc mouse embryos. Journal of Visualized Experiments. (2), e160(2006).
- Nonaka, S. Modification of mouse nodal flow by applying artificial flow. Methods in Cell Biology. 91, 287-297 (2009).
- Garcia, M. D., Udan, R. S., Hadjantonakis, A. K., Dickinson, M. E. Time-lapse imaging of postimplantation mouse embryos. Cold Spring Harbor Protocols. 2011 (4), 5595(2011).
- Crainiciuc, G., et al. Behavioural immune landscapes of inflammation. Nature. 601 (7893), 415-421 (2022).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved