Method Article
A Human Bone Marrow 3D Model to Investigate the Dynamics and Interactions Between Resident Cells in Physiological or Tumoral Contexts
In This Article
Summary
Here, we describe an easy-to-implement, standardized, microphysiological system that reflects the complexity of the human bone marrow's in vivo structure, providing a pertinent model to finely study a broad range of normal and pathological events.
Abstract
The medullary niche is a complex ecosystem that is essential to maintain homeostasis for resident cells. Indeed, the bone marrow, which includes a complex extracellular matrix and various cell types, such as mesenchymal stem cells, osteoblasts, and endothelial cells, is deeply involved in hematopoietic stem cell regulation through direct cell-cell interactions, as well as cytokine production. To closely mimic this in vivo structure and conduct experiments reflecting the responses of the human bone marrow, several 3D models have been created based on biomaterials, relying primarily on primary stromal cells. Here, a protocol is described to obtain a minimal and standardized system that is easy to set up and provides features of bone marrow-like structure, which combines different cell populations including endothelial cells, and reflects the heterogeneity of in vivo bone marrow tissue. This 3D bone marrow-like structure-assembled using calcium phosphate-based particles and human cell lines, representative of the bone marrow microenvironment-allows the monitoring of a wide variety of biological processes by combining or replacing different primary cell populations within the system. The final 3D structures can then either be harvested for image analysis after fixation, paraffin-embedding, and histological/immunohistochemical staining for cell localization within the system, or dissociated to collect each cellular component for molecular or functional characterization.
Introduction
Bone marrow (BM) is found within both the central cavities of axial and long bones and trabecular flat bones, and contains a variety of distinct cellular and non-cellular components. At the structural level, it consists of hematopoietic tissue islands (also called "hematons")1,2 and adipose cells surrounded by vascular sinuses interspersed within trabecular bone3. In long and flat bones, the blood supply to the bone and bone marrow are interconnected through an endosteal network of vessels4. Hidden in this solid protective network, the BM hosts very immature cells immersed in a spongy stroma and constitutes the location for the differentiation of resident mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs)5. Indeed, aside from the renewal of bone tissues through the production of osteoblasts, one of the main known functions of the BM resides in hematopoiesis development6.
The BM hematopoietic tissue includes a variety of cell types, namely HSCs, precursors, and more differentiated blood cells such as lymphocytes, neutrophils, erythrocytes, granulocytes, monocytes, and thrombocytes7. Hematopoietic cells are not randomly arranged in the BM space but display a particular organization within the hematopoietic niches, that include many cell types of different origins (osteoblasts, osteoclasts, MSCs, adipocytes, sinusoidal endothelium and perivascular stromal cells, immune cells, nervous cells, and distinct mature hematopoietic cells), forming a complex structure to ensure normal hematopoiesis8. The biological functions of the hematopoietic niche include regulation of HSC survival, adhesion, quiescence, homing, and differentiation through different mechanisms (cell-cell and cell-matrix interactions, production of soluble factors, hypoxia) in response to multiple physiological or non-physiological stresses3,6. Although these hematopoietic niches were initially perceived as homogeneous entities, technological advances such as single-cell and imaging analyses have progressively revealed their complexity, dynamics, and adaptive properties9,10,11.
The crucial need for replacing and reducing the use of animals for deciphering human physiological and pathological processes leads to new opportunities and challenges11,12. Among newly described in vitro tools, three-dimensional (3D) models that mimic the in vivo human BM better than classical two-dimensional (2D) cultures13,14,15,16,17 have been proposed. 3D modeling thus seems to be a promising approach to observe cell-cell and cell-matrix interactions, closer to those occurring in vivo. Using a variety of these models, some teams have demonstrated the survival and proliferation of HSCs18,19. Several 3D models are available, the most common approach being the use of scaffold-based 3D biomaterials such as hydrogels, colloids, or collagens, associated with MSCs taking advantage of their osteoblastic lineage differentiation capacities20,21,22. Nevertheless, no global consent has been reached to fulfill all requirements for studies on human cells in a user-friendly and standardized manner23, especially since these current 3D systems mainly rely on primary stromal cells, and thus suffer from limited access to primary samples, tissue availability, and heterogeneity.
Additionally, the endothelial cell compartment should be introduced as these cells represent a major component of cell-cell interactions and are involved in stem cell fate and BM disease development, both in leukemia24 and metastasis25. We previously reported that the addition of pathological elements in a standardized human 3D bone marrow model recapitulates features observed in patient samples such as extracellular matrix (ECM) alterations26. To better understand the dynamics and interactions between the human BM microenvironment and the resident cells, we provide a detailed protocol for a user-friendly and standardized system to build a minimal, well-organized, BM-like structure. This system is comprised of three human bone marrow cell types-endothelial cells and stromal cells, which represent the microenvironment, and HSCs. By using specific beads as a scaffold and an osteoblastic differentiation medium, the obtained 3D structure will mimic the human medullar niche, allowing a handy study of human bone marrow.
Protocol
NOTE: To prepare the bone marrow-like backbone structure, this protocol relies on combining beads with HMEC-1 endothelial cells and HS27A stromal cells on biphasic calcium phosphate (BCP) beads. The BCP beads will serve as a scaffold allowing, with a specific medium, the 3D structural shaping.
1. Bead preparation and sterilization
- Weigh 35-40 mg of BCP beads (HA/β-TCP 60/40; 45-75 µm) for each insert (small, cylindrical, plastic dish with a polycarbonate membrane at the bottom) in a 1.5 mL tube (see Table of Materials). After weighing the beads, bore a small hole with a clean needle through the top of the 1.5 mL tube to avoid the lid popping open during the autoclave cycle (121 °C for 60 min), and place the tubes in a vertical position in a closed sterile box .
2. 3D insert preparation under a sterile hood
- Put sterile polycarbonate cell culture inserts within the wells of a 6-well plate. Pour sterile BCP beads from a 1.5 mL tube into the insert. Wash the beads carefully by dripping 350 µL of 1x phosphate-buffered saline (PBS) inside the insert and then add 4.5 mL of 1x PBS to the well. Remove and discard the PBS either by using vacuum or a 5 mL pipette by placing the tip in a corner of the well. Repeat the washing step twice.
- Once the beads are washed, use 1x PBS supplemented with 10% fetal bovine serum (FBS) to block the system. Carefully add 350 µL of the blocking solution to the insert and 4.5 mL to the well and place the whole plate in an incubator at 37 °C, 5% CO2 overnight.
NOTE: Steps 2.1 and 2.2 help to minimize ion release from BCP beads before cell seeding.
3. Cell line harvesting and 3D maintenance
NOTE: After this incubation step, microenvironmental cells are added to form the 3D structure backbone. This system is based on the use of HMEC-1, human microvascular endothelial cells immortalized with large T antigen from SV40, and HS27A, human bone marrow stromal cells immortalized with human papillomavirus, owing to their ability to form a solid BM-like structure and to their compatibility with the inclusion of endothelial cells.
- Before adding cells to the system, prepare the following incubation media:
- For 50 mL of complete Osteo Differentiation Medium (ODM), mix 45 mL of Dulbecco's modified eagle medium (DMEM) + 5 mL of FBS + 0.5 mL of penicillin-streptomycin + 8 × 10-4 mg/L of dexamethasone + 2.16 g/L of beta-glycerophosphate + 44 mg/L of ascorbic acid.
- For 50 mL of complete HMEC-1 medium, mix 45 mL of MCDB 131 + 5 mL of FBS + 0.5 mL of penicillin-streptomycin + 1% of glutamine substitute + 1 mg/L of hydrocortisone + 10 µg/L of EGF.
- Remove the blocking solution from the system. Mix 1 × 106 HMEC-1 cells (representing the vascular compartment) and 2 × 106 HS27A cells (representing the mesenchymal stromal cell compartment) in a 15 mL tube and centrifuge for 5 min at 1,575 × g at room temperature. Discard the supernatant and add 175 µL of HMEC-1 medium (specific medium for HMEC-1 cell growth) with 175 µL of ODM (specific medium for HS27A osteodifferentiation).
- Pour 350 µL of this suspension containing 3 × 106 total cells inside each insert. Then, pour 4.5 mL of the combined media (2.25 mL of HMEC-1 medium + 2.25 mL of ODM) without cells into the wells of the plate. Gently aspirate and dispense the mixture several times with a 1 mL micropipette to homogenize the cells and BCP beads within the insert. Allot the whole mix to settle at the bottom of the insert.
NOTE: A mix preparation with one dead volume is recommended if several inserts are cultured. For instance, if four inserts are cultured, always prepare the volume required for five inserts. - Once the endothelial and mesenchymal cells are homogenized inside the insert, keep the 6-well plate inside an incubator at 37 °C, 5% CO2 for 3 weeks. Change the medium inside the insert and the well 2x a week by carefully removing the medium from the insert using a 1 mL micropipette and placing the tip at the inner insert wall to avoid disturbing the corner of the insert.
NOTE: The retrieved supernatant can be stored at -80 °C at any step for later protein quantification.
4. Addition of cells of interest into the system
NOTE: After 3 weeks of HMEC-1 and HS27A culturing, the osteodifferentiation of HS27A cells enabled the rigidification of the system. At this step, add hematological cells of interest (number of added cells can vary from 1 × 104 to 1 × 106) within the insert. The added cells were TF1 cells transfected with BCR-ABL oncogene and primary mononucleated CD34+ cells coming either from acute myeloid leukemia patients or healthy donors.
- Change the medium composition at this step, as the ODM compound is toxic for hematological cells and there is no need to continue adding it once the system rigidification is done. For instance, to support hematopoietic maintenance, substitute ODM medium with complete RPMI for hematopoietic cell lines (RPMI supplemented with 10% of FBS and 1% of penicillin-streptomycin) or IMDM for primary hematopoietic cells. Prepare a batch of 50% complete HMEC-1 medium and 50% complete RPMI/IMDM medium for the 3D medium (twice) weekly changes.
- Resuspend the hematological cells of interest in this combination medium and add it within the insert. If a drug treatment protocol is required, wait 24 h after adding the cells of interest before treating, giving them time to adhere to the bone-like structure.
- Adapt the period of 3D cell culture according to requirements. Refresh the medium (50% HMEC-1 complete medium + 50% RPMI complete medium) twice a week.
NOTE: Over the course of the 3D cell culture, some adherent cells might leak outside the insert into the bottom of the wells, increasing medium consumption. Check under the microscope for cell dispersion beyond the insert and replace the insert in a new 6-well plate under a sterile hood if needed.
5. 3D bone marrow-like experimental results
NOTE: Following 3D culturing, several outcomes can be achieved according to the objectives of the experiment.
- Protein analysis
NOTE: Over the course of the 3D experiment, the proteins secreted by the cells present in the bone marrow-like structure can be collected for further analyses.- When culture medium is changed 2x a week, store the retrieved supernatant from the inside of the insert at -80 °C to evaluate the production of proteins of interest within the system.
- Dissociating cells from the 3D structure (under a sterile hood if cells need to be sorted)
NOTE: The cells cultured in the 3D bone marrow-like system can be retrieved, sorted, and further analyzed.- At the end of the experiment, in a 15 mL tube, mix 4.5 mL of RPMI with 0.5 mL of FBS supplemented with 0.4 mg/mL of collagenase, and warm the solution at 37 °C for 10 min.
- Place the insert upside down (white membrane side on top) on a sterile 6-well plate cover to cut the membrane out properly. Use a sterile scalpel to cut the membrane from the sides, thus retrieving a white solid disc. Place the disc inside the warm 15 mL tube prepared in step 5.2.1.
- Place the tube in an active wheel in a 37 °C incubator and incubate for 10 min to allow digestion (in contact with collagenase). Apply the same incubation time for all membranes to avoid a skewed analysis.
- After 10 min, centrifuge the 15 mL tube for 5 min at 1,575 × g at room temperature, discard the supernatant, and resuspend the pellet in 5 mL of warm 1x PBS.NOTE: At this step, as the polycarbonate membrane generally floats in the supernatant fraction, it can be discarded by using a sterile pipette tip. If the membrane is still included in the pellet, leave it and remove it at the end of the next step.
- Resuspend the pellet in 300 µL of warm trypsin, vortex the tube for 10 s, and place it in a 37 °C water bath for 1 min.
- Stop the enzymatic digestion by adding 1 mL of warm pure FBS. With a 1 mL micropipette, mechanically aspirate and dispense the medium to completely dissociate the disc.
CAUTION: The aspiration/dispensing step must be very quick, otherwise the beads will sediment inside the tip. - Centrifuge the tube for 5 min at 1,575 × g. Resuspend the pellet in 3 mL of 1x PBS supplemented with 10% FBS.
- Leave the beads to sediment for 5 s before collecting the supernatant and filtering it through a 35 µm cell strainer placed on a collection tube. Centrifuge the retrieved filtrate for 5 min at 1,575 × g. Count the cells with trypan blue to evaluate the viability and proceed to flow cytometry analyses.
NOTE: The number of viable cells retrieved after trypan blue incubation is ~1-2 × 105 cells per digested insert.
- Flow cytometry labeling of retrieved cells
NOTE: The cells retrieved from the 3D bone marrow-like structure can be labeled and further analyzed by flow cytometry. Use a mix of antibodies to detect the different compartments after 3D dissociation. Here, CD45 (stains the hematological cells of interest), CD31 (stains the vascular compartment), and CD73 (stains the osteoblastic compartment) were used at 1 µL in 50 µL of PBS + 2% FBS. Keep all the prepared cells and antibodies on ice (4 °C) away from light during the whole process.- Resuspend the pellets in the antibody mix and incubate for 30-40 min on ice away from light.
- Add 1 mL of PBS + 2% FBS to wash the cells and centrifuge for 5 min at 1,575 × g.
- Resuspend the retrieved pellet in 200 µL of 1x PBS + 10% FBS supplemented with 4',6-diamidino-2-phenylindole (DAPI, diluted 1:2,000).
- Analyze the tubes in a cytometer using the following parameters: FSC 297 PnV; SSC 220 PnV; DAPI 400 PnV; CD45 APC 505 PnV. Use an FSC-H versus FSC-W graph plot to gate the singlet population. In the singlet fraction, use a DAPI-A versus FSC-A graph plot to determine the viable cells population (DAPI-negative). With the DAPI-negative population previously gated, use an APC-A versus FSC-A graph plot to determine the CD45-positive population.
- Fixation of the 3D bone marrow-like structure
NOTE: Fixation of the 3D BM-like structure is generally performed for immunohistochemistry or immunofluorescence to visualize cells in situ and for further specific staining.- To fix the BM-like structure, transfer the insert (step 4.3) from the old plate to a new 6-well plate filled with 1x PBS. Then, gently replace the medium inside the insert with 1x PBS.
NOTE: Do not flush the formed structure with PBS; the pressure of the liquid may impair the 3D structure. - Once the insert is washed, place it within a new 6-well plate and fill both the well and the insert with freshly-opened paraformaldehyde (PFA) 4% (diluted 16% in 1x PBS).
- Allow the fixation process to proceed for 4 h at room temperature away from light.
- At the end of the fixation process, wash the insert once with 1x PBS and store it at 4 °C away from light.
- Cut the membrane at the bottom of the insert as described in step 5.2.2 to retrieve the solid disk.
- Incubate the disk 4x for 48 h in EDTA (0.5 M, pH 8) in order to decalcify the structure.
- Embed the structure in paraffin and slice 4 µm thick sections.
- Using an automated immunostainer, incubate the sections with anti-CD45, anti-CD73, and Ki67 antibodies. Visualize the staining with an anti-rabbit or -mouse horse radish peroxidase (HRP) and with 3,3'-diaminobenzidine as a chromogenic substrate and counterstain with Gill's-hematoxylin.
- To fix the BM-like structure, transfer the insert (step 4.3) from the old plate to a new 6-well plate filled with 1x PBS. Then, gently replace the medium inside the insert with 1x PBS.
Results
Using this protocol, it is possible to recapitulate a minimal, in vitro, 3D, human BM-like microenvironment, from which viable cells from three different cell compartments can be retrieved (Figure 1). Indeed, after the desired exposure, 3D BM-like structures can be dissociated and the MSCs, endothelial cells, and hematopoietic cells can be evaluated/characterized using flow cytometry (Figure 2A). So far, the results suggest that it is possible to maintain hematological cells for at least 6 weeks within the 3D model, before retrieving viable cells (Figure 2B). However, future investigations are required to estimate the limit of this 3D model. In addition, if required, it is possible to substitute the HS27A cell line with primary Normal Bone Marrow (NBM) MSC or Acute Myeloid Leukemia (AML) MSC in this 3D BM-like model (Figure 2C) or replace the hematopoietic cell line with primary HSCs (Figure 2D). Moreover, when analyzing the interactions between cell compartments or the localization of specific markers of interest, 3D BM-like structures can be fixed and further processed with paraffin embedding and immunochemistry. For example, the analysis of CD45 (hematological) or CD73 (MSC) contents was performed using these 3D models (Figure 3).
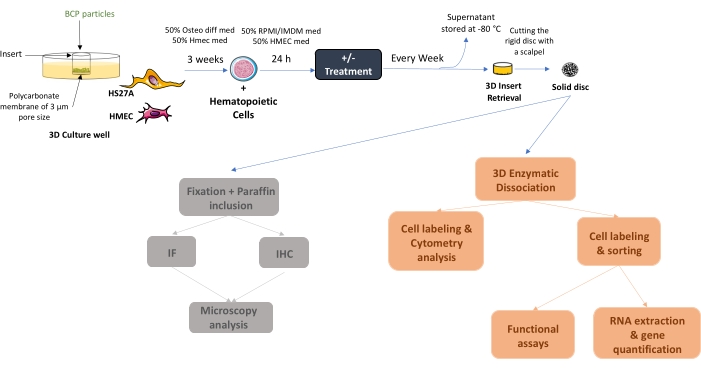
Figure 1: Schematic diagram of the initial setup of the human 3D BM-like system. After 3 weeks of co-culture, solid BM-like tissue can be collected or seeded with hematopoietic cells (as in the example) or other cell types. If required, once cells are adherent, treatment can be added and medium changes conducted twice a week. At the end of the experiment, BM-like structures can be either fixed and further processed for localization studies or dissociated to evaluate cell contents. Abbreviations: BCP = biphasic calcium phosphate; BM = bone marrow. Please click here to view a larger version of this figure.
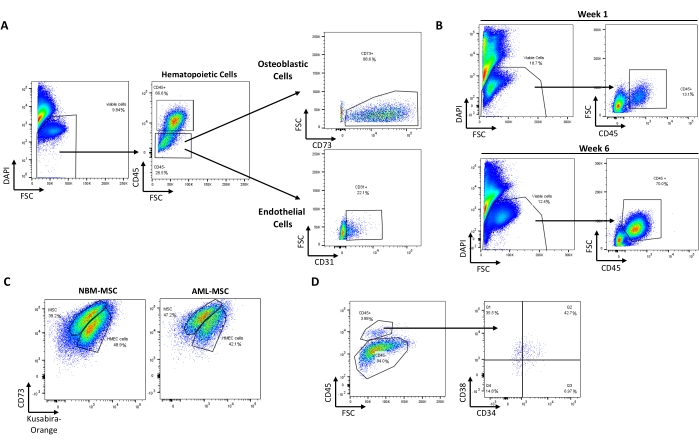
Figure 2: Recovery of viable cells from different compartments after culture in the BM-like 3D model. (A) Typical pattern of cell recovery from one dissociated BM-like structure with 9.84% of viable cells (DAPI-). Representative plots of hematopoietic (66.6% CD45+), MSC/osteoblast cells (88.6% CD73+ cells within the CD45- population), and endothelial cells (22.1% CD31+ cells within the CD45- population) recovered after 3D dissociation. (B) The 3D system allows maintenance of viable hematopoietic cells (CD45+) up to 6 weeks post culture. (C) 3D systems made of primary MSCs from NBM or AML alongside HMEC-1 (colored with Kusabira-Orange in this example) can maintain HSCs (3.88% CD45+ DAPI- cells) in situ (D). Abbreviations: BM = bone marrow; DAPI = 4',6-diamidino-2-phenylindole; MSC = mesenchymal stem cell; NBM = normal bone marrow; AML = acute myeloid leukemia; HSCs = hematopoietic stem cells; FSC = forward scatter. Please click here to view a larger version of this figure.
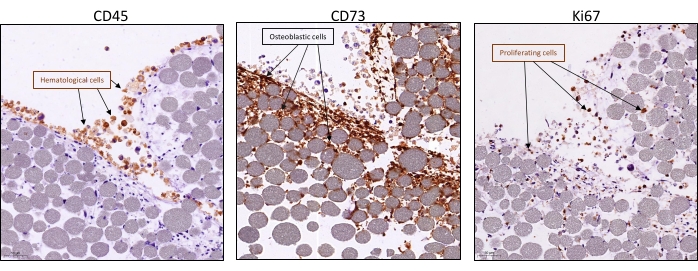
Figure 3: Visualization of cell interactions and proliferation within the BM-like 3D model. Systems were collected after 3 weeks of 3D co-culture with HS27A and HMEC-1 and the addition of hematopoietic cells after 1 week. Typical immunohistochemistry staining (visualized in brown) by IHC was visualized using an anti-rabbit or -mouse horse radish peroxidase and visualized with 3,3′-diaminobenzidine as a chromogenic substrate and counterstained with Gill's-hematoxylin and shows the presence of either hematopoietic cells with CD45 (left panel), stromal cells with CD73 (middle panel), or proliferation of all cells with KI67 (right panel). Scale bars = 50 µm. Abbreviations: BM = bone marrow; IHC = immunohistochemistry. Please click here to view a larger version of this figure.
Discussion
One of the current challenges faced by studies on human physiological and associated pathological issues is the lack of models accurately mimicking the complex functions of human organs. In the case of human BM 3D models, many models use hydrogels and partially reproduce the BM, illustrating the power of 3D culture conditions compared to classical 2D cultures in ensuring, for instance, a better osteoblastic differentiation27,28. Nevertheless, despite good reproducibility and ease of use, these systems do not mimic the human BM 3D structure and architecture, which may significantly impact further analyses. Technological advances have enabled scientists to better reproduce the native human osteoblastic niche environment using a perfusion-based bioreactor system to maintain some HSC properties29. The development of microfluidic devices has led to new approaches to reproduce a relevant bone marrow-like structure, but have so far only achieved limited features of the bone marrow, and therefore have restricted applications30,31,32,33.
Advances in material sciences have contributed to the development of a wide range of natural or synthetic biomaterials, which can be used to develop relevant 3D bone culture systems. However, such systems are sometimes difficult to develop in standard biological laboratories with no in-house skills in biophysics. Moreover, in most cases, these systems achieve a limited ability to mimic the 3D structure of the human bone, including the BM microenvironment, and have used large amounts of cytokines and growth factors, creating an artificially rich culture medium34.
The choice to induce osteoblastic differentiation rather than adipocytic differentiation was based on the fact that preosteoblasts and osteoblasts have been described as part of the endosteal niche35. This identified osteoblastic cells as requested components of both bone and bone marrow. Among the cellular components of the bone marrow, endothelial and stromal cells occupy a large proportion of the microenvironment. In addition, these two cell types produce structural non-cellular components of the extracellular matrix and cytokines involved in the regulation of homeostasis and differentiation, requested here to generate a calcified structure. Thus, this justifies the use of endothelial and stromal cells, and our choice of cell lines to allow easy access and increase reproducibility between labs. With the culture of the HMEC-1 line being more stable over time, this cell line was retained for the development of the 3D system. For stromal cells, we compared in 2D culture the osteoblast differentiation capacity of the stromal cell lines derived from normal bone marrow: HS5 and HS27A. We found that the HS5 line did not differentiate as much as the HS27A line in the 3D system generated with either cell line. In this regard, attempts to replace HS27A cells by the HS5 line have resulted in the production of a less rigid structure, suggesting that HS27A are more suitable.
Both HS27A and HMEC-1 cell lines were cultured in different media combinations, in which one elicits the best rigid structure and alignments of HMEC-1 along osteoblast structures so that the production of the extracellular matrix brings a relative rigidity to the basic structure. Analysis of the advancement of differentiation at D14 showed that the differentiation was more advanced at D21 (measurement of BSP, late osteoblastic marker), with a ratio 1:2 for HMEC-1:HS27A and with better results when 2 × 106 HS27A were introduced. Endothelial cells also have an important role in regulating homeostasis and differentiation (among other things through the supply of cytokines). The fact that HMEC-1 cells are maintained in this system indicates that they can at least fulfill this role, and this contributes to the legitimacy of our model. For the HMEC-1 endothelial line, it was important to introduce it early enough so that it could be properly integrated into the structure and with the hope that these cells could form alignments or even tubes within the structure. Co-culture tests of HS27A and HMEC-1 were carried out by introducing the latter at different stages: D0, D7, D14; no notable difference were observed, neither in the differentiation of the stromal cells nor in the maintenance or positioning of the endothelial cells. Thus, these cells were introduced at the beginning of the process. Hematopoietic cells were introduced at a time when osteoblastic differentiation would be advanced enough to favor cell-cell interactions. This choice was also conditioned by the fact that this osteoblastic differentiation is conditioned by the use of a medium, which according to our tests, does not fully support the maintenance of the hematopoietic cells. Hematological cells can be either cell lines or primary BM CD45 hematopoietic cells.
From this 3D model, we could envisage replacing each cell type by primary cells, normal or pathological, or even to add other cell types to boost biomimetic properties, such as immune cells, adipocytes, or fibroblasts26. In fact, the HS27A cell line has been replaced with primary BM MSCs with low passaging. Similarly, hematological cell lines were replaced with primary BM hematopoietic cells. However, the replacement of HMEC-1 cells by primary endothelial cells has not been tested yet. Currently, this model could still be improved in terms of vasculature representation, as even though endothelial cells are present and correctly interact with other cells from the microenvironment (e.g., hematopoietic cells), they do not form structured vessels, thus precluding flow perfusion to mimic functional blood vessels. Overall, this could progressively increase the complexity and representativeness of the current minimal 3D model. The addition of other cell types may facilitate studies investigating other issues, such as the importance of local BM inflammation or resistance to immunotherapy.
However, this 3D system needs 3 weeks before cells of interest, hematological cells or epithelial cancer cells if metastasis process is evaluated, can be added. Sterile conditions need to be maintained, as medium changes twice weekly can sometimes lead to medium contamination. Further, since some cells may migrate through the pores of the insert and begin to disperse into the well, leading to increased medium consumption, regular monitoring is recommended.
We described here a 3D scaffold allowing osteoblastic differentiation and developed a relevant, in vitro, 3D, human BM-like microenvironment. This system allows in situ analysis and ultimate live cell retrieval. This system provides a new and highly flexible tool, reproducible and user-friendly, with a broad range of applications to study interactions and mechanisms within the human BM microenvironment.
Disclosures
The authors declare no competing interests.
Acknowledgements
We thank P. Battiston-Montagne and A. Jambon from the CRCL cytometry platform and N. Gadot and C. Leneve from the Research Pathology Platform, Department of Translational Research and Innovation, Centre Léon Bérard. The authors are grateful to B. Manship for the English edition. This work was funded by Inserm and FI-LMC to V. M. S., and S. L. K. A. and L. B. were supported by a grant from Société Française d'Hématologie.
Materials
| Name | Company | Catalog Number | Comments |
| 3,3-Diaminobenzidine (DAB) Liquid Substrate System | MERCK SIGMA | D7304 | IHC staining |
| 5 mL Pipette | SARSTEDT | 861253001 | Medium change |
| Anti Mouse HRP | Thermofisher | 62-6520 | IHC secondary staining |
| Anti Rabbit HRP | Thermofisher | 31460 | IHC secondary staining |
| Ascorbic Acid 250 μM | MERCK SIGMA | A92902 | ODM medium |
| BCP | CaP Biomaterials LLC-US | 3D Beads | |
| Beta Glycerophosphate 10 mM | MERCK SIGMA | G9422 | ODM medium |
| CD31-BV711 | BD | 744078 | 3D endothelial Cell population labelling |
| CD34-APC | BD | 555824 | 3D immature hematological Cell population labelling |
| CD38-PE-Cy5 | BD | 555461 | 3D progenitor hematological Cell population labelling |
| CD45 | Miltenyibiotec | 130-110-637 | IHC staining of hematological cells |
| CD45-APC | BD | 555485 | 3D hematological Cell population labelling |
| CD45-BV500 | BD | 560777 | 3D hematological Cell population labelling |
| CD73 | Miltenyibiotec | 130-120-066 | IHC staining of stromal compartment |
| CD73-BV605 | BD | 563199 | 3D osteoblastic Cell population labelling |
| Cell Strainer for FACS equipment with 5 mL tube | FALCON | 352235 | Strainer and tube used to collect the cells and eliminate beads |
| Collagenase | MERCK SIGMA | C2674-1G | 3D enzymatic dissociation |
| Cytometry filtration tube | Thermofisher | 10585801 | 3D retrieved cells filtration |
| Cytometry tube | Thermofisher | 10100151 | 3D retrieved cells labelling |
| DAPI (Solution 12) | Chemometec | 910-3012 | 3D retrieved viable cells labelling |
| Dexamethasone | Thermofisher | A13449 | ODM medium |
| DMEM, high glucose, GlutaMAX Supplement, pyruvate 500 mL | Thermofisher | 31966021 | ODM medium |
| EGF | MERCK SIGMA | E9644-0.2MG | HMED-1 medium |
| Eppendorf 1.5 mL tube | SARSTEDT | 72696 | For beads autoclave and supernatant retieve |
| Falcon 15 mL | FALCON | 352096 | For 3D Dissociation |
| FBS | DUTSCHER | S1900-500 | To supplement culturing medium and stop trypsin action |
| Glutamax | Thermofisher | A1286001 | glutamine substitute for HMED-1 medium |
| Hematoxylin Solution, Gill No. 1 | MERCK SIGMA | GHS132-1L | IHC staining |
| HMEC-1 | ATCC | CRL-3243 | 3D endothelial Cell population |
| HS27A | ATCC | CRL-2496 | 3D osteoblastic Cell population |
| Hydrocortisone | MERCK SIGMA | H0135-1MG | HMED-1 medium |
| Insert: Nunc Polycarbonate Cell Culture Inserts in Multi-Well Plates | THERMO SCIENTIFIC NUNC | 140627 | To harvest cells and form a 3D Bone like structure |
| KI-67 IHC | Thermofisher | MA5-14520 | IHC staining of proliferative cells |
| MCDB 131 Medium, no glutamine | Thermofisher | 10372019 | HMED-1 medium |
| Micropipette (1,000 µL) | Eppendorf | 4924000088 | Medium change |
| PBS D 1x | Thermofisher | 14190169 | Cells/ Insert wash |
| Penicillin-Streptomycin (10,000 U/mL) | Thermofisher | 15140122 | To supplement culturing medium |
| PFA (Formaldehyde 16%) | EUROMEDEX | EM-15710 | 3D Fixation (dilution with PBS 1X) |
| RMPI 1640 medium, glutamax supplement | Thermofisher | 61870044 | Cuture medium |
| Scalpel | FISHER SCIENTIFIC | 11768353 | 3D membrane cutting |
| Six well plate | FALCON | 353046 | 3D culture |
| TRYPSIN-EDTA SOLUTION (1x) | Thermofisher | 25300096 | 3D enzymatic dissociation |
References
- Janel, A., et al. Bone marrow hematons: An access point to the human hematopoietic niche. American Journal of Hematology. 92 (10), 1020-1031 (2017).
- Blazsek, I., et al. The hematon, a morphogenetic functional complex in mammalian bone marrow, involves erythroblastic islands and granulocytic cobblestones. Experimental Hematology. 23 (4), 309-319 (1995).
- Morrison, S. J., Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature. 505 (7483), 327-334 (2014).
- Kopp, H. -G., Avecilla, S. T., Hooper, A. T., Rafii, S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology. 20 (5), 349-356 (2005).
- Hawley, R. G., Ramezani, A., Hawley, T. S. Hematopoietic stem cells. Methods in Enzymology. 419, 149-179 (2006).
- Gulati, G. L., Ashton, J. K., Hyun, B. H. Structure and function of the bone marrow and hematopoiesis. Hematology/Oncology Clinics of North America. 2 (4), 495-511 (1988).
- Dean, L. Blood and the Cells it Contains. Blood Groups and Red Cell Antigens. National Center for Biotechnology Information. , https://www.ncbi.nlm.nih.gov/books/NBK2263/ (2005).
- Kandarakov, O., Belyavsky, A., Semenova, E. Bone marrow niches of hematopoietic stem and progenitor cells. International Journal of Molecular Sciences. 23 (8), 4462(2022).
- Batsivari, A., et al. Dynamic responses of the haematopoietic stem cell niche to diverse stresses. Nature Cell Biology. 22 (1), 7-17 (2020).
- Tikhonova, A. N., et al. The bone marrow microenvironment at single-cell resolution. Nature. 569 (7755), 222-228 (2019).
- Tjin, G., et al. Imaging methods used to study mouse and human HSC niches: Current and emerging technologies. Bone. 119, 19-35 (2019).
- Low, L. A., Mummery, C., Berridge, B. R., Austin, C. P., Tagle, D. A. Organs-on-chips: into the next decade. Nature Reviews. Drug Discovery. 20 (5), 345-361 (2021).
- Ingber, D. E. Is it time for reviewer 3 to request human organ chip experiments instead of animal validation studies. Advanced Science. 7 (22), 2002030(2020).
- Walasek, M. A., van Os, R., de Haan, G. Hematopoietic stem cell expansion: challenges and opportunities. Annals of the New York Academy of Sciences. 1266 (1), 138-150 (2012).
- Discher, D., et al. Biomechanics: cell research and applications for the next decade. Annals of Biomedical Engineering. 37 (5), 847-859 (2009).
- Raic, A., Rödling, L., Kalbacher, H., Lee-Thedieck, C. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials. 35 (3), 929-940 (2014).
- Gamblin, A. L., et al. Osteoblastic and osteoclastic differentiation of human mesenchymal stem cells and monocytes in a miniaturized three-dimensional culture with mineral granules. Acta Biomaterialia. 10 (12), 5139-5147 (2014).
- Leisten, I., et al. 3D co-culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials. 33 (6), 1736-1747 (2012).
- Taqvi, S., Roy, K. Influence of scaffold physical properties and stromal cell coculture on hematopoietic differentiation of mouse embryonic stem cells. Biomaterials. 27 (36), 6024-6031 (2006).
- Chou, D. B., et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nature Biomedical Engineering. 4 (4), 394-406 (2020).
- Kotha, S. S., et al. Engineering a multicellular vascular niche to model hematopoietic cell trafficking. Stem Cell Research & Therapy. 9 (1), 77(2018).
- Marturano-Kruik, A., et al. Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proceedings of the National Academy of Sciences. 115 (6), 1256-1261 (2018).
- Panoskaltsis, N., Mantalaris, A., Wu, J. H. D. Engineering a mimicry of bone marrow tissue ex vivo. Journal of Bioscience and Bioengineering. 100 (1), 28-35 (2005).
- Behrmann, L., Wellbrock, J., Fiedler, W. Acute myeloid leukemia and the bone marrow niche-take a closer look. Frontiers in Oncology. 8, 444(2018).
- Kusumbe, A. P. Vascular niches for disseminated tumour cells in bone. Journal of Bone Oncology. 5 (3), 112-116 (2016).
- Voeltzel, T., et al. A minimal standardized human bone marrow microphysiological system to assess resident cell behavior during normal and pathological processes. Biomaterials Science. 10 (2), 485-498 (2022).
- Costantini, M., et al. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication. 8 (3), 035002(2016).
- Chatterjee, K., et al. The effect of 3d hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening. Biomaterials. 31 (19), 5051-5062 (2010).
- Bourgine, P. E., et al. In vitro biomimetic engineering of a human hematopoietic niche with functional properties. Proceedings of the National Academy of Sciences. 115 (25), 5688-5695 (2018).
- Carvalho, M. R., Reis, R. L., Oliveira, J. M. Mimicking the 3D biology of osteochondral tissue with microfluidic-based solutions: breakthroughs towards boosting drug testing and discovery. Drug Discovery Today. 23 (3), 711-718 (2018).
- de al Puente, P., et al. 3D tissue-engineered bone marrow as a novel model to study pathophysiology and drug resistance in multiple myeloma. Biomaterials. 73, 70-84 (2015).
- Bersini, S., et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 35 (8), 2454-2461 (2014).
- Mansoorifar, A., Gordon, R., Bergan, R., Bertassoni, L. E. Bone-on-a-chip: microfluidic technologies and microphysiologic models of bone tissue. Advanced Functional Materials. 31 (6), 2006796(2021).
- Nelson, M. R., et al. A multi-niche microvascularized human bone marrow (hBM) on-a-chip elucidates key roles of the endosteal niche in hBM physiology. Biomaterials. 270, 120683(2021).
- Galán-Díez, M., Kousteni, S. The osteoblastic niche in hematopoiesis and hematological myeloid malignancies. Current Molecular Biology Reports. 3 (2), 53-62 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved