Method Article
Assessment of Chemical Toxicity in Adult Drosophila Melanogaster
* These authors contributed equally
In This Article
Summary
This protocol describes an efficient and inexpensive method that uses liquid media to assess the effects of chemical toxicants on the viability of adult Drosophila melanogaster.
Abstract
Human industries generate hundreds of thousands of chemicals, many of which have not been adequately studied for environmental safety or effects on human health. This deficit of chemical safety information is exacerbated by current testing methods in mammals that are expensive, labor-intensive, and time-consuming. Recently, scientists and regulators have been working to develop new approach methodologies (NAMs) for chemical safety testing that are cheaper, more rapid, and reduce animal suffering. One of the key NAMs to emerge is the use of invertebrate organisms as replacements for mammalian models to elucidate conserved chemical modes of action across distantly related species, including humans. To advance these efforts, here, we describe a method that uses the fruit fly, Drosophila melanogaster, to assess chemical safety. The protocol describes a simple, rapid, and inexpensive procedure to measure the viability and feeding behavior of exposed adult flies. In addition, the protocol can be easily adapted to generate samples for genomic and metabolomic approaches. Overall, the protocol represents an important step forward in establishing Drosophila as a standard model for use in precision toxicology.
Introduction
Humans are constantly exposed to chemicals from a variety of sources, including air1, food2, water3,4, medications5, cleaning agents6, personal care products7, industrial chemicals7, and building materials7. Moreover, thousands of new chemicals are introduced each year8, many of which are not properly vetted for health and environmental safety. This lack of adequate chemical safety testing stems in part from an over-reliance on mammalian models, such as mice and rats. While such rodent models are informative, chemical safety testing in these systems is expensive, time-consuming, and often causes unacceptable levels of suffering to the test animal9.
The financial and ethical burdens associated with mammalian chemical safety testing, as well as the time-consuming nature of mammalian studies, are major contributing factors to the paucity of data surrounding new chemicals. To address this issue, the U.S. Environmental Protection Agency (EPA), the European Chemicals Agency (ECHA), Health Canada, and other agencies are implementing measures that incorporate new approach methodologies (NAMs) into regulatory frameworks10, thus placing North American and European policy in line with international goals to replace, reduce, and refine the use of animals (the 3Rs principal)11,12,13,14. NAMs encompass a variety of assays primarily based on in vitro and in silico models that provide a mechanistic understanding of chemical toxicity instead of observing adversity inflicted on mammalian test species, thereby increasing the rate of data generation for chemical risk assessment while still producing high fidelity outputs15. However, these methods are not yet proven to safeguard against systemic toxicity, including the disruption of vital biological processes involving interorgan communication and endocrine signaling. Further, they cannot account for the bioaccumulation of chemicals within specific tissues, the ability of individual compounds to be absorbed and secreted, and the interplay between behavior and chemical exposure.
Due to the limitations of in vitro and computational models, the successful use of NAMs to reduce or replace mammalian models should also include invertebrate in vivo models, such as the fruit fly, Drosophila melanogaster. Previous studies in the fly have demonstrated that this organism is well suited for studying the conserved genetic pathways that protect animal cells against toxic molecules16,17,18,19,20,21,22. Moreover, the fly shows remarkable genetic similarity to humans, including functional homologs to over 65% of human diseases23,24,25 and an even greater conservation of important functional pathways26. These features, combined with their relatively short life cycle, low maintenance cost, and readily observable behavioral responses, make Drosophila well-suited for use as a toxicological model27,28,29,30. Moreover, flies have much higher throughput than rodent models and capture effects on metabolism, physiology, and hormone signaling that are not readily detectable by other non-organismal NAMs9.
The protocol described here represents a framework for testing the effects of chemical exposure on adult Drosophila. The method is designed to be efficient, inexpensive, and reproducible, while also minimizing the time researchers must be in contact with the test chemical and accommodating sample collection for metabolomics and other omics approaches. The protocol is optimized for testing a single chemical per experiment, but can easily accommodate other experimental parameters, such as varied solvents or combinations of chemicals.
Protocol
NOTE: Wear nitrile gloves for all steps in this protocol. Wear a laboratory coat, eye protection, and/or respirators, as per the safety data sheets for each evaluated chemical.
1. Vial and humidity chamber preparation
NOTE: Steps 1.1-1.5 can be completed at any time before beginning the other experimental sections. Nitrile gloves must be worn at all times during vial preparation to prevent contamination.
- Stack four sheets of grade 1 cellulose chromatography paper (see Table of Materials) and cut them into 2 in wide strips. Punch out flower-shaped filter paper inserts using a 1.5 in paper punch containing a flower-shaped die.
- Use a 22 mm x 220 mm unvarnished wooden dowel to push the filter paper to the bottom of a 28.5 mm diameter polypropylene vial. Confirm that the stack of filter paper is securely located at the bottom of the vial.
- Store the prepared vials in plastic or cardboard trays and place the trays in large (~280 mm x 240 mm) plastic bags until use.
- Construct a humidity chamber by cutting a 120 mm x 280 mm hole in the plastic lid of a 606.24 mm x 225.42 mm x 403.22 mm plastic tub (see Table of Materials). Glue mesh over the hole to allow airflow.
- Cut a section of the plastic grid from louvered ceiling light panels (originally 610 mm x 1220 mm) to fit in the bottom of the plastic tub used in step 1.4.
NOTE: A variety of different plastic tubs and plastic/metal grid materials can be used to build a humidity chamber.
2. Fly husbandry
- Start cultures of adult flies (minimum of 30 adults) in glass milk bottles containing standard Bloomington Drosophila Stock Center (BDSC) media31. Close the bottles with a rayon plug wrapped in a delicate task wipe. Do not crowd the bottles.
NOTE: The number of required bottles depends on the number of chemical exposures being conducted and the genotype of the test strain. Normally, several hundred flies can be obtained from a single bottle when using robust and fecund stocks. The standard assay uses Oregon-R wild-type flies (BDSC stock #2057), but any genotype(s) of interest can be used with this protocol. Remember that compromised genotypes with low fecundity and/or viability require increased bottle cultures. - Incubate the trays of culture bottles at 25 °C with approximately 60% humidity and a 12:12 h light:dark cycle until the third larval instar or early pupal stages are observed. This stage is identifiable by the presence of larvae wandering up the sides of the bottle and the appearance of pupae on bottle walls.
NOTE: Only handle flies during the lights-on period of the 12:12 h light:dark cycle. For robust stocks, bottles reach this stage after 3-4 days under the described conditions.- Remove adult flies and determine the liquidity of the media. If the media flows along the side of the bottle when inverted, the medium is too liquidy. Insert a delicate task wipe or a rayon ball into the bottom of the bottle to solidify the fly food.
- When the flies begin to eclose, clear all adults from the bottles and allow pupae to continue to eclose for 48 h. At this point, any adults cleared from the culture bottles can be either transferred to new bottles to propagate the stocks (see step 2.1) or discarded.
- Transfer flies that eclose in the 48 h period to new bottles containing standard BDSC media. Age for 3 days.
NOTE: The adults in these bottles are 3-5 days old and can be used in the lethality and feeding experiments. The bottles used to age adult flies will contain eggs and larvae. As a result, these bottles can be used to propagate the next generation of flies.
3. Preparation of flies for chemical exposure
- Anesthetize 5-7-day-old flies with CO2. Sort the anesthetized flies by sex using their genitalia. For assistance with anesthetizing and sexing flies, see reference28.
- Place groups of either 20 male or 20 female flies into vials containing standard BDSC media. Close the vial with a rayon plug and store the male and female vials separately. Mark the vials containing female flies with a stripe. Leave the vials with male flies unmarked to avoid accidentally mixing sexes.
NOTE: The number of vials that must be prepared in step 3.2 is dictated by the size of the exposure experiments. As described in steps 5.3 and 5.6, a typical range finding experiment for a single test chemical requires a minimum of 40 vials of males and 40 vials of females. A standard dose-response curve experiment, described in steps 7.4 and 7.8, requires a minimum of 63 vials of males and 63 vials of females. - Store the vials for 48 h in an incubator at 25 °C at approximately 60% humidity and with a 12:12 h light:dark cycle. During this time, prop the tray of sorted vials at a 60° angle to prevent flies from getting stuck in the food while recovering from the anesthesia.
NOTE: This step allows flies to recover from the CO2 anesthesia. Do not anesthetize the flies again during the remainder of the exposure protocol. - After the 48 h recovery period, add 0.75 mL of sterile purified water to the vials prepared in step 1.3. The number of vials prepared in this step should be identical to the number of vials set up in step 3.2.
- Transfer sorted, sex-matched flies to the starvation vial by opening the glass vial containing the flies from step 3.2, putting it into the mouth of the prepared plastic vial, and then tapping the bottom of the plastic vial against a benchtop. Close the plastic vial with a cellulose acetate plug (commonly known as a flug).
- Record any flies lost in this transfer (transfer to records of the starting number of flies for each vial later).
- Mark starvation vials containing female flies with a stripe. Leave the vials with male flies unmarked to avoid accidentally mixing sexes.
- Prepare the humidity chamber for overnight exposure. See steps 1.4-1.5 for a description of how to build this chamber.
- Place six standard paper towels in the bottom of the humidity chamber. Soak the paper towels with 100 mL of water.
- Put the plastic grid (step 1.5) over the wet towels to ensure the vials do not come into contact with the saturated paper towels.
- Place the trays of starvation vials in a horizontal position within the humidity chambers. Place the humidity chambers in a 25 °C incubator (at approximately 60% humidity) overnight.
NOTE: Ideally, the overnight starvation period lasts approximately 16 h; however, the timing can be adjusted for individual lab schedules.
4. Preparation of stock solutions
NOTE: Flies are fed test chemicals in a yeast-sucrose liquid media. This section describes preparing stock solutions of concentrated feeding media and test chemicals.
- Prepare a 4x yeast-sucrose solution containing 16% sucrose and 6% yeast extract (m/v) (see Table of Materials) dissolved in sterile purified water. Autoclave the solution on a liquid cycle for the appropriate sterilization time (e.g., 40 min for 1 L).
NOTE: The solution can be made in bulk and stored in aliquots at -20 °C. Thaw individual aliquots 1 day prior to use by placing them in a 4 °C refrigerator. - Prepare a stock of the test chemical. For the initial experiment, the stock solution should be made at the highest concentration so that the chemical can be completely dissolved in water.
NOTE: Solvents other than water can be used to dissolve the test chemical. See the discussion regarding caveats for using alternative solvents. - Prepare a 100x blue dye stock solution by dissolving 1 g of FD&C Blue No. 1 (see Table of Materials) in 10 mL of sterile purified water.
NOTE: The blue dye stock solution can be made in bulk and stored in aliquots at 4 °C.
5. Preparation of exposure vials: Range finding experiment
NOTE: Steps 5 and 6 of the protocol are designed to identify the lowest dose of test chemical that induces 100% lethality and the highest dose that fails to induce a lethal phenotype. If these concentrations are already determined by previous experimentation, see steps 7 and 8 for calculating a dose-response curve. Exposure media must be prepared immediately prior to adding flies to the exposure vials.
- Label eight 15 mL centrifuge tubes as follows: (i) no chemical, (ii) highest concentration, (iii) 1:2, 1:10, 1:20, 1:100, 1:200, and 1:1,000.
- Prepare the exposure media in the labeled centrifuge tubes from step 5.1 by first adding 2.5 mL of 4x yeast/sucrose stock solution to all eight labeled tubes.
- Add 7.5 mL of sterile purified water to the tube labeled "no chemical". This dilution is the negative control.
- Add 7.4 mL of the test chemical stock solution to the tube labeled "highest concentration". Add 100 μL of sterile purified water to this tube, so the final volume is 10 mL. Calculate and record the molarity of the test chemical in this solution.
NOTE: The addition of 100 μL of sterile purified water to the tube ensures that the chemical concentration in this step is identical to that used in step 5.5, where a blue dye stock solution is added to the exposure media as a method for assessing feeding behavior. - Add the appropriate amount of test chemical stock solution and sterile purified water to the remaining tubes. The final volume of each tube must be 10 mL. The final concentration of test chemicals in these tubes relative to the "highest concentration" tube must be 1:2, 1:10, 1:20, 1:100, 1:200, and 1:1,000.
- Prepare and label eight exposure vials for each individual concentration of exposure media generated in step 5.1. There should be eight sets of vials (64 vials total) containing the following labels: no chemical, highest concentration, 1:2, 1:10, 1:20, 1:100, 1:200, and 1:1,000.
- Pipette 0.75 mL of exposure media prepared in step 5.2.3 into the corresponding set of exposure vials.
- Label eight 5 mL individual tubes as follows: (i) no chemical, (ii) highest concentration, (iii) 1:2, 1:10, 1:20, 1:100, 1:200, and 1:1,000.
- Prepare blue dye exposure media by first mixing 500 μL of 4x yeast/sucrose stock solution and 20 μL of blue dye stock solution in eight labeled 5 mL tubes.
- Add 1.48 mL of sterile purified water to the tube labeled "no chemical". This dilution is the negative control.
- Add 1.48 mL of the test chemical stock solution to the tube labeled "highest concentration". No water is added to this tube. Calculate and record the molarity of the test chemical in this solution.
- Add the appropriate amount of test chemical stock solution and sterile purified water to the remaining tubes. The final volume of each tube should be 2 ml. The final concentration of test chemical in these tubes relative to the "highest concentration" tube should be 1:2, 1:10, 1:20, 1:100, 1:200, and 1:1,000.
- Prepare and label two exposure vials with each individual concentration of blue exposure media. There should be eight sets of vials (16 vials total) containing the following labels: no chemical, highest concentration, 1:2, 1:10, 1:20, 1:100, 1:200, and 1:1,000. The word "blue" should also be written on these vials.
- Pipette 0.75 mL of blue exposure media into the corresponding set of blue exposure vials.
6. Fly chemical exposure: Range finding experiment
- Prepare chemical exposure vials using the vials of overnight starved flies from step 3.7 as follows:
- Transfer four vials of starved female flies (20 flies per vial) into four exposure vials of each chemical concentration. Label these vials "female". Use the same transfer method described in step 3.5.
- Transfer four vials of starved male flies (20 flies per vial) into four exposure vials of each chemical concentration. Label these vials "male". Use the same transfer method described in step 3.5.
- Prepare chemical exposure vials containing blue dye using the vials of overnight starved flies from step 3.7 as follows:
- Transfer one vial of starved female flies (20 flies per vial) into one blue exposure vial for each chemical concentration. Label this vial "female". Use the same transfer method described in step 3.5.
- Transfer one vial of starved male flies (20 flies per vial) into one blue exposure vial for each chemical concentration. Label this vial "male". Use the same transfer method described in step 3.5.
- Record the number of flies that are present in each vial after transfer and note the number that died or escaped. Normally, all 20 flies should survive overnight starvation and transfer.
- Place the exposure vials horizontally in freshly prepared humidity chambers (see step 3.6 for humidity chamber preparation). Place the chambers in a 25 °C incubator with approximately 60% humidity and a 12:12 h light:dark cycle.
- Examine the exposure vials at 24 and 48 h after the start of the chemical exposure. Count and record the number of dead flies in each vial at each time point.
NOTE: Death is used as the readout for this assay, but the protocol can be adapted to examine other phenotypes. Exposed flies are commonly collected at these timepoints for transcriptomic and metabolomic studies. - Examine the blue exposure vials at 24 h after the start of the chemical exposure. Use the following techniques to determine if exposed flies consumed the blue exposure media:
- Examine the vial walls for indications of blue feces, which appear as small dots on the side of the exposure vial as well as on the flug.
- Anesthetize the flies with CO2 and examine the abdomens for the presence of blue dye.
NOTE: Normally, blue exposure vials are analyzed after 24 h. However, this step could be performed at 48 h, instead. Flies that have eaten normally have a blue stripe through the abdomen, indicating that the exposure media has entered the gut. - Examine the flies for abnormal feeding behaviors, such as regurgitation, crop distension, and breakdown of the intestinal barrier function (indicated by the appearance of blue dye throughout the organism rather than just limited to the GI tract, commonly referred to as smurfing32,33).
- Discard all contaminated vials, filter paper, flugs, and flies in appropriate chemical waste containers. If live flies remain in the vials, freeze the vials to kill the flies prior to discarding them in the proper waste container.
NOTE: Disposal of exposure vials and flies is dictated by the chemical(s) being analyzed in the experiment. Always follow the chemical safety procedures outlined on the chemical safety data sheet. If concentrations used in the range finding experiment kill flies at the lowest dose, repeat section 6 using a dilution series, beginning with the lowest concentration that killed 100% of animals.
7. Preparation of exposure vials: Generating a dose-response curve
NOTE: The protocol outlined in steps 5 and 6 is designed to broadly determine the chemical concentration required to elicit a phenotype. Steps 7 and 8 of the protocol are used to calculate an accurate dose-response curve.
- Calculate the test chemical concentrations that must be analyzed to generate a dose-response curve using the following method:
- Determine the lowest concentration of the test chemical that kills 100% of exposed flies at 48 h.
- Determine the highest concentration of test chemical that has no effect on viability at 48 h.
- Calculate an additional four concentrations that are equally distributed between the concentrations determined in steps 7.1.1 and 7.1.2.
- Label nine individual 15 mL centrifuge tubes with the molarity of the following concentrations: (i) no chemical, (ii) concentration determined in step 7.1.1, (iii) concentration determined in step 7.1.2, (iv) concentrations determined in 7.1.3, (v) twice the concentration determined in step 7.1.1, (vi) a 1:2 dilution of concentration determined in step 7.1.2.
NOTE: Including the concentrations that are twice the concentration determined in 7.1.1 and a 1:2 dilution of that determined in step 7.1.2 is important for accurately calculating the dose-response curve. - Prepare the exposure media by first adding 2.5 mL of 4x yeast/sucrose stock solution to nine labeled individual 15 mL centrifuge tubes prepared in step 7.2.
- Add 7.5 mL of sterile purified water to the tube labeled "no chemical". This dilution is the negative control.
- Add the appropriate amount of test chemical stock solution and sterile purified water to the remaining tubes. The final volume of each tube must be 10 mL. The final concentration of chemical within an individual tube should be equal to that written on the outside of the tube.
- Prepare and label 12 exposure vials for each concentration of exposure media generated in step 7.2. There should be nine sets of vials (108 vials total).
- Pipette 0.75 mL of exposure media into the corresponding set of exposure vials.
NOTE: Steps 7.6 to 7.8 are optional. If the test chemical concentrations prepared in step 7.2 are known to not affect feeding behavior, these steps can be skipped. - Prepare and label nine different 5 mL tubes for blue exposure media with the same series of concentrations used in step 7.2.
- Prepare blue dye exposure media by first mixing 500 μL of 4x yeast/sucrose stock solution and 20 μL of blue dye stock solution.
- Add 1.48 mL of purified sterile water to the tube labeled "no chemical". This dilution is the negative control.
- Add the appropriate amount of test chemical stock solution and purified sterile water to the remaining tubes. The final volume of each tube must be 2 mL. The final concentration of chemical within an individual tube should be equal to that written on the outside of the tube.
- Prepare and label two exposure vials for each individual concentration of blue exposure media. There should be nine sets of vials (18 vials total), with each set of vials labeled with the concentrations listed in step 7.2. The word "blue" should also be written on these vials.
- Pipette 0.75 mL of blue exposure media into the corresponding set of blue exposure vials.
8. Fly chemical exposure: Generating a dose-response curve
- Prepare chemical exposure vials using the vials of overnight starved flies from step 3.7 as follows:
- Transfer six vials of starved female flies (20 flies per vial) into six exposure vials of each chemical concentration. Label these vials "female". Use the same transfer method described in step 3.5.
- Transfer six vials of starved male flies (20 flies per vial) into six exposure vials of each chemical concentration. Label these vials "male". Use the same transfer method described in step 3.5.
- Prepare chemical exposure vials containing blue dye using the vials of overnight starved flies from step 3.7 as follows:
- Transfer one vial of starved female flies (20 flies per vial) into one blue exposure vial for each chemical concentration. Label this vial "female". Use the same transfer method described in step 3.5.
- Transfer one vial of starved male flies (twenty flies per vial) into one blue exposure vial for each chemical concentration. Label this vial "male". Use the same transfer method described in step 3.5.
- Record the number of flies present in each vial after transfer. Normally, all 20 flies should survive the overnight starvation and transfer, but ensure any that died or escaped during transfer are subtracted from the total.
- Place the exposure vials horizontally in freshly prepared humidity chambers (see step 3.6 for humidity chamber preparation). Place the chambers in a 25 °C incubator with approximately 60% humidity and a 12:12 h light:dark cycle.
- Examine the exposure vials at 24 and 48 h after the start of the chemical exposure. Count and record the number of dead flies in each vial at each time point.
- Examine the blue exposure vials at 24 h after the start of the chemical exposure. Use the method outlined in step 6.6 to assess changes in feeding behavior.
- Discard all contaminated vials, filter paper, flugs, and flies in appropriate chemical waste containers. If live flies remain in the vials, freeze the vials to kill the flies prior to discarding in the proper waste container.
NOTE: Disposal of exposure vials and flies is dictated by the chemical(s) being analyzed in the experiment. Always follow the chemical safety procedures outlined on the chemical safety data sheet.
9. Calculating a dose-response curve
- Use the Benchmark Dose Software (BMDS, version 3.2; see Table of Materials) or other similar software to analyze the data34. The following describes the workflow using the BMDS software.
- Click on the Data tab of BMDS and click on insert new data set. Input the number of samples in the dataset, click on dichotomous, and then click on create dataset. Enter each replicate as an individual row in the dataset.
- Input the dose in the first column of the generated table, the starting number of flies excluding any that died or were lost prior to step 8.3) in the second column, and the number of flies that died from chemical exposure in the third column.
- Click on the Main tab of BMDS.
- Click on the drop-down arrow for the Select Model Type menu and click on Dichotomous.
- Click on enable for the dataset from step 9.2 in the Data Sets table.
- Click on the boxes for the desired models for analysis in the MLE and Alternatives table.
NOTE: The Frequentist Restricted Dichotomous Hill model was primarily used. - Click on Run analysis. The program will generate an output file with the lethality curve(s) and additional details about the model(s).
Results
The fly has long served as a model in studies for determining sodium arsenite (NaAsO2) toxicity35,36,37,38. To demonstrate the efficacy of the protocol, male and female flies were exposed to NaAsO2, with the goal of comparing these results with earlier studies. Using the methodology described above, adult Oregon-R (BDSC stock #2057) males and females were exposed to a range of NaAsO2 concentrations (0, 0.01, 0.02, 0.1, 0.2, 1, and 2 mM) and scored for lethality 48 h after the start of exposure (Figure 1A,B).
The purpose of this initial analysis was to identify the approximate range of concentrations that would allow a more precise characterization of NaAsO2 toxicity. In subsequent experiments, concentrations were selected (0, 0.2, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, and 5 mM) that more precisely defined the NaAsO2 dose-response curve (Figure 1C,D). Note that the resulting analysis examined several concentrations that induced 100% lethality. Data were analyzed using the Environmental Protection Agency's publicly available Benchmark Dose Software version 3.2.0.125. The data were modeled as "dichotomous" and the Dichotomous Hill model was used for subsequent analyses. Based on this model, the final LD10, LD25, and LD50 of male flies fed NaAsO2 were 0.30 mM, 0.50 mM, and 0.65 mM, respectively. For female flies, these values were slightly higher, with an LD10 of 0.30 mM, an LD25 of 0.65 mM, and an LD50 of 0.90 mM. Overall, the values obtained using this method are similar to those previously reported for arsenic toxicity in the Drosophila melanogaster35,36,37,38, thus validating the methodology.
In addition to the six replicates used to calculate the dose-response curve, male and female flies were also fed NaAsO2 exposure solutions that contained 1% FD&C blue, which is easily visible in the digestive tract using light microscopy. Based on the presence of blue dye within the intestine of NaAsO2-fed flies, both male and female flies continued to feed 24 h after the beginning of the chemical exposure, regardless of the NaAsO2 concentration present within the liquid media (Figure 2). However, the ingested food was observed to be occasionally regurgitated at doses above 0.2 mM for females and 0.5 mM for males (Figure 2). These findings suggest that regurgitation could serve a key role in the Drosophila response to arsenic poisoning.

Figure 1: Dose-response curves for male and female Drosophila treated with NaAsO2 for 48 h. All graphs show the estimated proportions of dead flies at each NaAsO2 concentration tested based on the Dichotomous Hill model. (A,B) A broad range of NaAsO2 concentrations were tested to approximate the dose at which each sex of fly begins to die. (A) shows the male data, and (B) shows the female data. N = 4 vials, with 20 flies per vial. (C,D) A narrower range of NaAsO2 concentrations was tested to determine precise doses at which 10%, 25%, and 50% of each sex of flies died. These doses are indicated to the right of each graph. (C) shows the male data, and (D) shows the female data. N = 6 vials, with 20 flies per vial. Please click here to view a larger version of this figure.
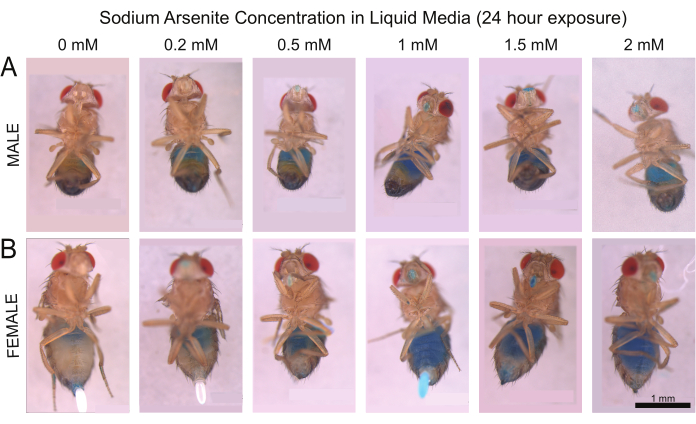
Figure 2: Representative results from the blue dye assay of male and female Drosophila treated with NaAsO2 for 24 h. Micrographs show flies fed increasing concentrations of NaAsO2. Row (A) shows male flies, and row (B) shows female flies, with the concentration of NaAsO2 increasing from left to right. The abdomen shows a small amount of blue near the thorax at low concentrations, indicating that exposure media entered the gut. At higher concentrations, blue dye begins to accumulate around the mouth, suggesting that exposure media is being regurgitated. The scale bar is 1 mm. Please click here to view a larger version of this figure.
Discussion
The fruit fly Drosophila melanogaster is emerging as a powerful system for NAMs16,18,19,21. By leveraging the unparalleled genetic resources available to the fly community, combined with recent advances in genomics and metabolomics, chemical safety studies using Drosophila are capable of quickly identifying the molecular mechanisms by which individual compounds interfere with metabolism, physiology, and cell signaling (for example, see39). This inexpensive protocol is designed to rapidly define dose-response curves and subsequently generate samples for RNA-seq and metabolomics analysis. Moreover, this flexible protocol can be adapted for use with any genotype and can accommodate many classes of chemicals.
A notable aspect of this protocol is the choice of liquid food used in the chemical exposure, which is based on a previous study, but differs from the solid media used by most toxicological studies of Drosophila18,22. This specific liquid media was selected to reflect the nutritional content of the standard, solid BDSC media that the flies are also fed in this protocol, to ensure the flies receive consistent nutrition. The simplicity of liquid feeding media has many advantages. Liquid media is easier to handle than solid food, which needs to be either melted and resolidified or reconstituted from powder. Liquid media also increases the system's throughput, ensures even chemical distribution throughout the feeding media, and decreases the time spent working with hazardous compounds. Additionally, the media does not require solutions to be heated, which facilitates the testing of volatile test compounds. Finally, because of the relatively few components included in the food solution, undesirable side reactions are minimized between the test chemical and other dietary components. The yeast used in the food is also inactive, further limiting the reactivity of the feeding medium. However, please note that the method is not suitable for testing developmental or larval toxicity.
Some of the materials used in the protocol can be substituted, such as using glass fly vials rather than polypropylene. However, the materials used were selected to be both inert and disposable to avoid unwanted chemical reactions between reagents and chemical exposures that could result from cleaning glassware.
The use of liquid food necessitates a vehicle for food delivery. Cellulose acetate filter paper was selected for this purpose due to its flexibility and inert nature28. Other researchers used similar protocols but with other vehicles, such as delicate task wipes or glass fiber filter29,30. The cellulose acetate filter paper suited these needs because it is an inert vehicle which can be cut to the ideal shape to fit it into the bottom of the fly vials without large gaps between the paper and vial wall, preventing death due to flies becoming stuck in media or the vehicle itself.
An important limitation of this system is that the maximum testable concentration of a chemical is tied to the solubility of the chemical. Non-water-soluble compounds require an additional solvent, which can lead to additional or synergistic effects with the chemical of interest. This can also create situations in which it is not possible to prepare stock solutions that are concentrated enough to achieve the desired endpoint in all organisms, therefore limiting analysis of the resulting data31. To address this, chemicals with low water solubility can be tested by adding up to 0.5% dimethyl sulfoxide to the food solution. Other solvents could be used as well, but additional research is needed for each solvent of interest to determine the maximum acceptable solvent concentration within the solution to maximize solubility while minimizing solvent effects on the organism.
Extensive characterization of the olfaction response in Drosophila has described how flies avoid consuming toxic compounds40,41, leading to reduced feeding on treated media. The blue dye assay addresses this phenomenon by allowing researchers to efficiently screen the feeding behaviors of the flies fed each concentration of experimental chemical42,43,44. The presence or absence of blue in the fly's gastrointestinal tract indicates if the fly has been eating the toxicant-containing medium. Although more sophisticated methods of assessing fly feeding behaviors exist, such as the Fly Liquid-Food Interaction Counter45, this qualitative method is better suited for higher-throughput screening.
A notable aspect of this protocol is that it has been optimized for a 48 h exposure period without the need to transfer flies or add additional liquid to the exposure vial. Using a humidity chamber and placing the chambers in an incubator kept at high humidity prevented the filter paper containing the feeding media from drying out during this timeframe. The protocol can be adapted for longer exposure durations, but the method must be adjusted to ensure that the filter paper does not become dry and cause significant changes in solution concentration or lethality due to desiccation.
Finally, an important characteristic of this protocol is that it can readily accommodate genetic variants, which allows researchers to utilize the vast array of genetic tools for Drosophila to expand these preliminary studies on wild-type organisms to better understand mechanisms of chemical action in vivo. In this regard, the protocol outlined above could be easily modified to complement a previously described JoVE protocol by Peterson and Long that allows for toxicological analysis of wild-caught flies18.
Because of the wide variety of previous studies on the toxicity of sodium arsenite in Drosophila32,33,34,35,36, Oregon-R flies were treated with this compound to demonstrate the efficacy of our system. Male flies exhibited an LD50 of 0.65 mM, and females exhibited an LD50 of 0.90 mM. This aligns with previous studies of sodium arsenite-treated adult Drosophila. For example, Goldstein and Babich37 found that 50% of flies (mixed sexes) died after 7 days of exposure to 0.5 mM NaAsO2. Although this is a slightly lower dose than was presently observed, the differences between their methods and this method (including the use of solid exposure media, a longer time scale, and mixed sexes) likely account for this difference. Importantly, both methods resulted in overall similar LD50 values.
Observations from experiments using this protocol can be used to find genetic and molecular targets for subsequent behavioral or mechanistic studies. The exposure method can also be used to treat Drosophila for sampling for metabolomics and proteomics, making this protocol well suited to the growing field of precision toxicology (modeled from the precision medicine field46). In this regard, exposed flies can be collected after step 8 for subsequent genomic and metabolomics analysis. Samples collected in step 8 can then be processed, as described by Li and Tennessen47, starting with step 3.
Ultimately, the data acquired from the experiments described above, as well as any subsequent metabolomics and proteomics data, would ideally be used in cross-species comparisons. As previously noted26, such cross-species studies are powerful and capable of determining how individual chemicals interfere with conserved biological pathways. Thus, the protocol described above can be used to find evolutionary commonalities in response to individual toxicants across phyla and help inform chemical safety regulation.
Disclosures
No conflicts of interest to disclose.
Acknowledgements
We thank our staff for help with the testing and optimization of this protocol: Ameya Belamkar, Marilyn Clark, Alexander Fitt, Emma Rose Gallant, Ethan Golditch, Matthew Lowe, Morgan Marsh, Kyle McClung, Andy Puga, Darcy Rose, Cameron Stockbridge, and Noelle Zolman. We also thank our colleagues from the Precision Toxicology Group, particularly our Exposure Group counterparts, for helping to identify the goals of the protocol.
This project received funding from the European Union's Horizon 2020 Research and Innovation program under Grant Agreement No. 965406. The work presented in this publication was performed as part of the ASPIS Cluster. This output reflects only the authors' views, and the European Union cannot be held responsible for any use that may be made of the information contained therein. This publication was also made possible with support from the Indiana Clinical and Translational Sciences Institute, which is funded in part by Award Number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Portions of this project were supported by funds from Indiana University awarded to JRS and the PhyloTox consortium. JMH and EMP were supported by NIH award P40OD018537 to Bloomington Drosophila Stock Center.
Materials
| Name | Company | Catalog Number | Comments |
| 1.5 inch flower lever action craft punch | Bira Craft | HCP-115-024 | |
| 15 mL Centrifuge Tubes | VWR | 89039-666 | High-Performance Centrifuge Tubes with Flat or Plug Caps, Polypropylene, 15 mL |
| 2 ml Tubes | VWR | 16466-044 | Micro Centrifuge Tube with Flat Screw-Cap, conical bottom |
| 5 ml Tubes | VWR | 60818-576 | Culture Tubes, Plastic, with Dual-Position Caps |
| 50 mL Centrifuge Tubes | Corning | 430290 | 50 mL polypropylene centrifuge tubes, conical bottom with plug seal cap |
| Benchmark Dose Software version 3.2 | U.S. Environmental Protection Agency | ||
| Cardboard trays | Genesee Scientific flystuff | 32-122 | trays and dividers for narrow vials |
| CO2 gas pads | Genesee Scientific flystuff | 59-114 | FlyStuff flypad, CO2 anesthetizing apparatus |
| Combitips advanced, 50 mL | Eppendorf | 0030089693 | Combitips advanced, Biopur, 50 mL, light gray, colorless tips |
| Cotton balls | Genesee Scientific flystuff | 51-101 | Cotton balls, large, fits narrow vials |
| Delicate task wipes | Kimtech | 34155 | Kimtech Science Kimwipes Delicate Task Wipes, 1 Ply / 8.2" x 4.39" |
| Drosophila Vial Plugs, Cellulose Acetate (aka, Flugs) | VWR | 89168-888 | Wide |
| FD&C Blue No. 1 | Spectrum Chemical | FD110 | CAS number 3844-45-9 |
| Flies | BDSC | Stock #2057 | OregonR wildtype |
| Gloves (nitrile) | Kimtech | 55082/55081/55083 | Kimtech purple nitrile exam gloves, 5.9 mil, ambidextrous 9.5" |
| Grade 1 CHR cellulose chromatography paper | Cytvia | 3001-917 | Sheet, 46 x 57 cm |
| Mesh for humidity chamber | |||
| Multipette / Repeater (X) stream | Eppendorf | 022460811 | Repeater Xstream |
| Plastic grate | Plaskolite | 18469 (from lowes) | Plaskolite 24 in x 48 in 7.85 sq ft louvered ceiling light panels, cut down to fit in rubbermaid tubs |
| Plastic trays for glass vials | Genesee Scientific flystuff | 59-207 | Narrow fly vial reload tray |
| Polypropylene Drosophila Vial | VWR | 75813-156 | Wide (28.5 mm) |
| Rubbermaid tubs | Rubbermaid | 3769017 (from Lowes) | Rubbermaid Roughneck Tote 10 gallon 18" L x 12" W x 8 1/2" H |
| Sucrose ultra pure | MP Biomedicals, Inc. | 821721 | |
| Tube racks for wide-mouthed tubes | Thermo scientific | 5970-0230 | Nalgene Unwire Test tube racks, for 30 mm tubes |
| Water Purification System | Millipore Milli-Q | ZMQ560F01 | Millipore Milli-Q Biocel Water Purifier |
| Yeast extract | Acros Organics | 451120050 | CAS number 84604-16-0 |
References
- United States Occupational Safety and Health Administration. Construction industry: OSHA safety and health standards (29 CFR 1926/1910). United States Occupational Safety and Health Administration. , (2022).
- Chemical, Metals, Natural Toxins & Pesticides Guidance Documents & Regulations. United States Food & Drug Administration. , Available from: https://www.fda.gov/food/guidance-documents-regulatory-information-topic-food-and-dietary-supplements/chemical-metals-natural-toxins-pesticides-guidance-documents-regulations (2022).
- Drinking Water Contaminant Candidate List (CCL) and Regulatory Determination. United States Food & Drug Administration. , Available from: https://www.epa.gov/cci (2022).
- Administration Bottled Water/Carbonated Soft Drinks Guidance Documents & Regulatory Information. United States Food & Drug Administration. , Available from: https://www.fda.gov/food/guidance-documents-regulatory-information-topic-food-and-dietary-supplements/bottled-watercarbonated-soft-drinks-guidance-documents-regulatory-information (2022).
- United States Congress. Federal Food, Drug, and Cosmetic Act. United States Congress. 21, 301-392 (1934).
- Determining if a Cleaning Product is a Pesticide Under FIFRA. Environmental Protection Agency. , Available from: https://www.epa.gov/pesticide-registration/determining-if-cleaning-produt-pesticide-under-fifra (2022).
- United States Congress. Toxic Substances Control Act of 1976. H.R. 12440. Library of Congress, United States. , 1640-1707 (1976).
- Wang, Z., Walker, G. W., Muir, D. C. G., Nagatani-Yoshida, K. Toward a global understanding of chemical pollution: a first comprehensive analysis of national and regional chemical inventories. Environmental Science Technology. 54 (5), 2575-2584 (2020).
- Pandey, U. B., Nichols, C. D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacological Reviews. 63 (2), 411-436 (2011).
- New Approach Methods Work Plan. United States Environmental Protection Agency. , Available from: https://www.epa.gov/system/files/documents/2021-11/nams-work-plan_11_15_21_508-tagged.pdf (2021).
- Hartung, T. From alternative methods to a new toxicology. European Journal of Pharmaceutics and Biopharmaceutics. 77 (3), 338-349 (2011).
- Mahony, C. Building confidence in non-animal methods: Practical examples of collaboration between regulators, researchers and industry. Computational Toxicology. 10, 78-80 (2019).
- Kavlock, R. J., et al. Accelerating the pace of chemical risk assessment. Chemical Research in Toxicology. 31 (5), 287-290 (2018).
- European Chemicals Agency. New Approach Methodologies in Regulatory Science, Proceedings of the Scientific Workshop. European Chemicals Agency. , Helsinki, Finland, 19-20 April 2016 (2016).
- Wambaugh, J. F., et al. New approach methodologies for exposure science. Current Opinion in Toxicology. 15, 76-92 (2019).
- Rand, M. D. Drosophotoxicology: The growing potential for Drosophila in neurotoxicology. Neurotoxicology and Teratology. 32 (1), 74-83 (2010).
- Ong, C., Yung, L. Y. L., Cai, Y., Bay, B. H., Baeg, G. H. Drosophila melanogaster as a model organism to study nanotoxicity. Nanotoxicology. 9 (3), 396-403 (2015).
- Peterson, E. K., Long, H. E. Experimental protocol for using Drosophila as an invertebrate model system for toxicity testing in the laboratory. Journal of Visualized Experiments. (137), e57450(2018).
- Rand, M. D., Montgomery, S. L., Prince, L., Vorojeikina, D. Developmental toxicity assays using the Drosophila model. Current Protocols in Toxicology. 59, 11-20 (2014).
- Misra, J. R., Horner, M. A., Lam, G., Thummel, C. S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes & Development. 25 (17), 1796-1806 (2011).
- Rocha, J. B. T. Drosophila melanogaster as a promising model organism in toxicological studies. Archives of Basic and Applied Medicine. 1 (1), 33-38 (2013).
- Affleck, J. G., Walker, V. K. Drosophila as a model for developmental toxicology: using and extending the drosophotoxicology model. Methods in Molecular Biology. 1965, 139-153 (2019).
- Ugur, B., Chen, K., Bellen, H. J. Drosophila tools and assays for the study of human diseases. Disease Models & Mechanisms. 9 (3), 235-244 (2016).
- Chien, S., Reiter, L. T., Bier, E., Gribskov, M. Homophila: human disease gene cognates in Drosophila. Nucleic Acids Research. 30 (1), 149-151 (2002).
- Wangler, M. F., et al. Model organisms facilitate rare disease diagnosis and therapeutic research. Genetics. 207 (1), 9-27 (2017).
- Colbourne, J. K., et al. Toxicity by descent: A comparative approach for chemical hazard assessment. Environmental Advances. 9, 100287(2022).
- Hales, K. G., Korey, C. A., Larracuente, A. M., Roberts, D. M. Genetics on the fly: a primer on the Drosophila model system. Genetics. 201 (3), 815-842 (2015).
- Markstein, M. Drosophila Workers Unite! A Laboratory Manual for Working with Drosophila. , Available from: http://marksteinlab.org/wp-content/uploads/2019/01/MicheleMarkstein-DrosophiliaWorkersUnite-PREPRINT-JAN2019.pdf (2018).
- Vang, L. L., Medvedev, A. V., Adler, J. Simple ways to measure behavioral responses of Drosophila to stimuli and use of these methods to characterize a novel mutant. PLoS One. 7 (5), 37495(2012).
- Nichols, C. D., Becnel, J., Pandey, U. B., Byfield, F. Methods to assay Drosophila behavior. Journal of Visualized Experiments. (61), e3795(2012).
- Bloomington Drosophilia Stock Center. BDSC Cornmeal Food. , Available from: https://bdsc.indiana.edu/information/recipes/bloomfood.html (2022).
- Martins, R. R., McCracken, A. W., Simons, M. J. P., Henriques, C. M., Rera, M. How to catch a Smurf? - ageing and beyond… in vivo assessment of intestinal permeability in multiple model organisms. Bio-Protocol. 8 (3), 2722(2018).
- Rera, M., et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metabolism. 14 (5), 623-634 (2011).
- Benchmark Dose Software (BMDS) (Build 3.3; Model Library Version 2022.10) [Computer Software]. United States Environmental Protection Agency. , Available from: https://www.epa.gov/bmds/download-bmds (2022).
- Ortiz, J. G., Opoka, R., Kane, D., Cartwright, I. L. Investigating arsenic susceptibility from a genetic perspective in Drosophila reveals a key role for glutathione synthetase. Toxicological Sciences. 107 (2), 416-426 (2009).
- Pickett, A. D., Patterson, N. A. Arsenates: effect on fecundity in some Diptera. Science. 140 (3566), 493-494 (1963).
- Goldstein, S. H., Babich, H. Differential effects of arsenite and arsenate to Drosophila melanogaster in a combined adult/developmental toxicity assay. Bulletin of Environmental Contamination and Toxicology. 42 (2), 276-282 (1989).
- Polak, M., Opoka, R., Cartwright, I. L. Response of fluctuating asymmetry to arsenic toxicity: support for the developmental selection hypothesis. Environmental Pollution. 118 (1), 1928(1928).
- Zhou, S., et al. A Drosophila model for toxicogenomics: Genetic variation in susceptibility to heavy metal exposure. PLoS Genetics. 13 (7), 1006907(2017).
- Anholt, R. R. H. Chemosensation and evolution of Drosophila host plant selection. iScience. 23 (1), 100799(2020).
- Depetris-Chauvin, A., Galagovsky, D., Grosjean, Y. Chemicals and chemoreceptors: ecologically relevant signals driving behavior in Drosophila. Frontiers in Ecology and Evolution. 3, 41(2015).
- Aryal, B., et al. Protocol for binary food choice assays using Drosophila melanogaster. STAR Protocols. 3 (2), 101410(2022).
- Shimada, I., Nakao, M., Kawazoe, Y. Acute differential sensitivity and role of the central nervous system in the feeding behavior of Drosophila melanogaster. Chemical Senses. 12 (3), 481-490 (1987).
- Tanimura, T., Isono, K., Takamura, T., Shimada, I. Genetic dimorphism in the taste sensitivity to trehalose in Drosophila melanogaster. Journal of Comparative Physiology. 147 (4), 433-437 (1982).
- Ro, J., Harvanek, Z. M., Pletcher, S. D. FLIC: high-throughput, continuous analysis of feeding behaviors in Drosophila. PLoS One. 9 (6), 101107(2014).
- National Research Council. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. National Academy of Sciences. , National Academies Press. US. (2011).
- Li, H., Tennessen, J. M. Preparation of Drosophila larval samples for gas chromatography-mass spectrometry (GC-MS)-based metabolomics. Journal of Visualized Experiments. (136), e57847(2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved