Method Article
Biomechanical Changes Related to Low Back Pain: An Innovative Tool for Movement Pattern Assessment and Treatment Evaluation in Rehabilitation
* These authors contributed equally
In This Article
Summary
This study shows the role of an innovative tool for assessing and treating biomechanical alterations in low back pain (LBP) patients. Three LBP patients demonstrated improved pain intensity and functional independence post-assessment. The technology aids in tailored rehabilitation strategies, offering insights into LBP biomechanics for personalized interventions.
Abstract
Low back pain (LBP) is a highly prevalent disorder frequently related to biomechanical alterations. Movement pattern assessments have a role in the rehabilitation management of patients with LBP; however, a precise assessment is challenging in routine clinical settings. Thus, this study aims to assess the biomechanical alterations related to LBP through the development and application of an innovative assessment tool named CameraLab. Patients with LBP were assessed through a video analysis system. The movement pattern assessment tool includes a touchscreen interface and four high-velocity cameras, enabling real-time data acquisition during movement assessments. The cameras capture dynamic movements, facilitating a thorough examination of motor function. A video analysis software application is employed for precise angle assessments and joint tracking. Three patients with LBP were assessed, demonstrating positive results in pain intensity, functional independence, and overall well-being. The integration of advanced technology highlighted the movement pattern alterations and contributed to tailored rehabilitation strategies. The study offers a paradigm shift toward precision rehabilitation. This innovative approach provides valuable insights into the biomechanical changes related to LBP, fostering a deeper understanding for clinicians and paving the way for effective personalized interventions in the management of LBP.
Introduction
Low back pain (LBP) is a complex and prevalent musculoskeletal condition severely affecting physical functioning and Health-related Quality of Life (HR-QoL)1,2. LBP is a growing global public health problem, consistently ranking as a major cause of disability and limitations in function in daily life. According to the Global Burden of Disease (GBD) 2021 study, the prevalence of LBP is increasing, estimating nearly 619 million people worldwide in 2020. The study highlights that LBP represents a substantial portion of years lived with disability, with prevalence observed primarily in individuals aged 45 to 64 years3. Due to the aging of the population, its prevalence is expected to rise in the future decades while growing research is currently focusing on innovative approaches to improve the management of this condition4,5,6. The 2019 GBD analysis further confirms these findings, indicating that LBP remains a prevalent condition in several regions of the world, with a strong impact on HR-QoL7. Projections suggest that without effective intervention, the prevalence and burden of LBP will continue to grow, necessitating comprehensive global approach for prevention and management3,7.
While the optimal therapeutic strategy is typically based on the precise pathophysiology of LBP, different therapeutic approaches have been suggested to address the multifaceted management of this disabling condition8,9,10,11,12. The WHO Rehabilitation Guide provides a comprehensive framework for global rehabilitation practices, emphasizing their critical role in managing chronic LBP13. These guidelines underline the need for an integrated and personalized approach to the patient, addressing the biopsychosocial aspects of chronic pain management. This involves a coordinated effort among multidisciplinary healthcare professionals to provide non-surgical interventions based on scientific evidence and tailored to the individual needs of each patient. A comprehensive approach is essential to reduce variability in care, improve quality of life, and improve overall outcomes for those with LBP. The guidance also highlights the importance of accessibility and equity in rehabilitation services, ensuring that interventions are feasible and acceptable in different contexts, thus supporting universal health coverage and improving global public health13.
In this context, it is interesting to notice that patients with LBP are frequently characterized by crucial biomechanical changes that should be precisely addressed for an effective rehabilitation approach14,15,16. These alterations might include deviations in spinal alignment17, muscle imbalances18, joint stiffness or hypermobility19, aberrant movement patterns20, asymmetries in muscle activation12, and compromised neuromuscular control21,22. As a result, identifying and addressing these specific biomechanical changes are crucial for tailoring rehabilitation programs to target the underlying mechanisms contributing to LBP and facilitating optimal recovery outcomes23,24.
In this context, assessment methods for movement patterns might include motion inertial sensors, force plates, standardized observational tests, and qualitative observation criteria25,26,27,28,29,30. Motion inertial sensors, while offering portability and ease of use, have limitations primarily related to data accuracy and reliability. Their measurements can be affected by sensor drift, orientation errors, and signal noise, leading to inaccuracies in movement analysis29. Additionally, motion inertial sensors may have limited capacity to assess complex movement patterns accurately, especially in dynamic activities, including rapid movements or changes in direction20. Force plates, although valuable for quantifying ground reaction forces and kinetics during movement, have limitations regarding their spatial and temporal resolution30. They may not provide detailed information on movement quality or kinematic patterns and are primarily focused on assessing forces exerted on the ground rather than the movement patterns30. On the other hand, qualitative observation criteria, while useful for capturing qualitative aspects of movement, lack standardization and may vary between observers lacking in standardization and reliably27,28. Interestingly, the recent review by van Dijk et al.20 underlined that only specific domains of movement quality (such as range of motion (ROM) and gate analysis) were effectively assessed by objective methods in patients with LBP and significantly differed by the general population.
As a result, objective and quantifiable methods for movement assessment are lacking, and several challenges still affect both the intervention and monitoring processes for patients with LBP20. Moreover, the barriers to the effective integration of these tools into routine clinical practice further improve the challenges associated with effectively addressing LBP conditions.
Taken together, this evidence suggests that there remains a substantial gap in knowledge regarding digital instruments designed for evaluating movement quality during functional exercises. Furthermore, the implications of integrating precise movement assessment analysis into the rehabilitation process have yet to be fully characterized.
Therefore, here we present a case series introducing CameraLab system, an innovative digital solution that provides objective data about movement pattern analysis in patients with LBP. In some cases, instrumental examinations with X-Rays have little indication regarding rehabilitation implications in patients with LBP. In this case, the functional assessment with motion capture could fill this gap and provide answers to rehabilitation needs31. In this case series, we showed the effective integration of the innovative assessment tool into the comprehensive rehabilitation management of patients with LBP, underlining the functional and objective data achieved with this technological solution in order to enhance the precision and effectiveness of clinical rehabilitation practice in people with LBP.
Protocol
Before data collection, all patients included were provided with an informed consent form to review and sign, ensuring their understanding and agreement to participate in the study. Researchers granted the patient's privacy throughout all the study procedures, and they maintained compliance with ethical principles outlined in the Declaration of Helsinki32.
1. Organization of CameraLab setting
- Turn on the touch screen of the interactive monitor, the central control hub of the video analysis system (see Figure 1 for further details), and push the power button.
- Position of the four high-velocity cameras according to the distances indicated in Figure 2.
- Connect the four cameras to the interactive monitor: insert the network cables into their corresponding doors.
- Start the camera interface for viewing images in real-time. Click on the icon Video Analysis System to open the software.
2. Initial patient interview
- Provide the informed consent form for review and signature.
- Collect and note down agraphic and anthropometric data.
- Position the patient in the center of the video analysis system with four high-velocity cameras at 3 m each from the patient according to the distances indicated in Figure 2.
3. Assessment with functional movement screen (FMS) in accordance with current guidelines33,34
- Ask the patient to perform a warm-up.
- Low-intensity cycling: Ask the patient to pedal while maintaining the seat height to allow for easy achievement of 0° knee extension. Adjust the resistance to prevent fatigue throughout the 12 min duration.
- Lower limb stretching exercise: Ask the patient to lie in a supine position. Ask the patient to hug one thigh at a time to his chest with his hands clasped behind it. Then, perform 3 sets of 10 repetitions of knee extension on each leg.
- Core activation: Instruct the patient to start from a supine position with hips flexed and feet flat on the ground. Ask the patient to perform 15 hip extensions, reaching the bridge position for 3 sets, with a 15-s rest between sets.
- Administer to the patient the FMS Test (cameras off), including deep squats, Hurdle steps, Inline lunges, shoulder mobility, active straight leg raises, trunk stability push-ups, and rotatory stability, in accordance with the FMS guidelines33,34.
4. System acquisition
- Ask the patient to perform two different repetitions of the movements to gain confidence with the motor pattern of the test. Subsequently, ask the patient to perform 2 trials recorded with the innovative assessment tool. Include the movements mentioned below.
- Front squat, no hand: Ask the patient to assume the starting position by positioning the feet approximately shoulder width apart and aligned on the sagittal plane. Have the patient position arms extended forward with a bar resting on the arms. Then, ask the patient to descend as far as possible into a squat position while keeping the torso upright, keeping the heels and bar in place. Hold the downward position for a count of one, then return to the starting position.
- Lower Body Motor Control Screen (LB-MCS) (for each side): Ask the patient to assume the position, arms stretched forward and standing only on the foot of the side being evaluated, the contralateral lower limb is kept with the knee extended and with the foot not resting on the ground. Then, ask the patient to descend as far as possible into a squat position while keeping the torso upright and the foot resting in position. Hold the position down for a count of one, then return to the starting position.
- Press the Start button to start recording. The system starts data acquisition until the end of the exercise.
- Press Stop to stop the data acquisition. The system provided a video file stored in a specific folder.
5. Data analysis on interactive monitor
- Create the patient's folder on the interactive monitor with the recorded videos: right-click on the desktop to open the Desktop menu, choose New Folder, type the name of the new folder, and press Return.
- Open the video selected (Front squat no hand and LB-MCS for right side and LB-MCS for left side).
- For front squat, no hand trial, follow steps 5.2.1.1-5.2.1.8.
- Select the frame from the video generated in lateral views at the point of maximum descent.
- Start video annotation tool for image analysis by double clicking on the Own icon.
- Insert the frame into the report.
- Select the joints in the lateral frame (shoulder, hip, knee, and ankle) by touching the interactive monitor and drawing the skeleton of the limb and trunk axis (see Figure 4). The video annotation tool automatically gives target angles (hip and knee).
- Give scores based on the cut-off (see Table 1) in steps 5.2.1.6-5.2.1.7.
- Lower limb control score: Give a score of 0 if the hip-foot axis does not match the patella with the knee residing medial to the hip-foot axis, give a score of 1 if the hip-foot axis does match the patella laterally, and give a score of 2 if the hip-foot axis does match the patella medially.
- Motor strategy score. Give a score of 0 if active knee flexion > 110° and hip flexion > 100°, give a score of 1 if active knee flexion > 110° or hip flexion > 100°, and give a score of 2 is given if active knee flexion ≤ 110° and hip flexion ≤ 100°.
- Add the lower limb control score and the motor strategy score to calculate the total row score.
- For LB-MCS for right side and left side, follow steps 5.2.2.1-5.2.2.11.
- Select the frame from the video generated in both lateral and frontal views at the point of maximum descent.
- Start video annotation tool for image analysis by double clicking on the Own icon.
- Insert the frame into the report.
- Select the joint in the frontal frame (trunk axis, hips, and ankle of the side on the floor) by touching the interactive monitor and drawing the skeleton of the limb and trunk axis (see Figure 5).
- Select the joint in the lateral frame (shoulder, hip, knee, and ankle) by touching the interactive monitor and drawing the skeleton of the limb and trunk axis (see Figure 5). The video annotation tool automatically gives target angles (hip and knee).
- Give scores based on the cut-off (see Table 1) in steps 5.2.2.8-5.2.2.10.
- Lower limb control score: Give a score of 0 if the hip-foot axis does not match the patella with the knee residing medial to the hip-foot axis, give a score of 1 if the hip-foot axis does match the patella laterally, and give a score of 2 if the hip-foot axis does match the patella medially.
- Pelvic tilt score: Give a score of 0 if the pelvic angle does tilt ≥ 15° compared to the horizontal plane, give a score of 1 if the pelvic angle does tilt between 10°-15° compared to the horizontal plane, and give a score of 2 if the pelvic angle does tilt ≤ 10° compared to the horizontal plane.
- Trunk control score: Give a score of 0 if the deviation of the column segment from the perpendicular plane ≥ 15°, give a score of 1 if the deviation of the column segment from the perpendicular plane is between 10°-15°, and give a score of 2 if the deviation of the column segment from the perpendicular plane ≤ 10°.
- Motor strategy score. Give a score of 0 if active knee flexion > 110° and hip flexion > 100°, give a score of 1 if active knee flexion > 110° or hip flexion > 100°, and give a score of 2 if active knee flexion ≤ 110° and hip flexion ≤ 100°.
- Calculate the total row score, which is the sum of the lower limb control score, pelvic tilt score, trunk control score, and motor strategy Score.
- For front squat, no hand trial, follow steps 5.2.1.1-5.2.1.8.
- Process indications and return the test results to the patient.
Figure 1 shows the schematic representation of the protocol.
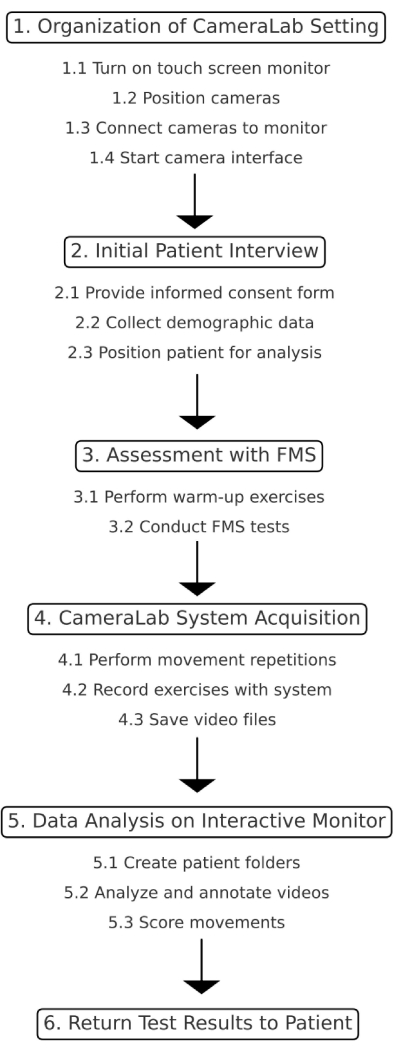
Figure 1: Schematic representation of the protocol. This figure illustrates the step-by-step process of the study protocol. Please click here to view a larger version of this figure.
Results
Study design and ethics
This manuscript has been written following the case reporting (CARE) structure and reporting guidelines, and the CARE checklist is available as Supplementary File 1. Eligible subjects are men and women with LBP, aged 18 to 60, who undergo physiotherapy to improve their condition and return to a daily life free from constant discomfort and pain that prevent the normal performance of ADLs.
Participants' inclusion criteria were: a) patients with LBP of any type; b) referred pain less than 4/10 on the numeric rate scale (NRS); c) patients who have already completed a standard rehabilitation cycle and have been referred for further rehabilitation cycles for the purpose of improving motor function; d) Patients must be able to perform a squat movement and control the hip hinge movement. Patients' exclusion criteria were: a) Physical limitations that may preclude testing; b) Previous vertebral fractures; c) A body mass index (BMI) of 30 or greater.
Three patients were included in this prospective case series and were assessed by a multidisciplinary team involving an expert physician specialized in Physical and Rehabilitation Medicine and a physiotherapist with years of expertise in LBP management. The patients were affected by LBP with different etiology and were assessed after a standard rehabilitation program, with the video analysis system and standard assessment outcomes including numeric rating scale (NRS)35; short-form 12-item health survey (SF-12)36, Roland Morris disability questionnaire (RM)37; Tampa scale of kinesiophobia (TSK)38. Functional movement screen (FMS) tests, initially developed for athletes, can be effectively applied to assess movement limitations and guide physical therapy interventions that emphasize movement and exercise-based approaches to LBP patients, even those with conditions like scoliosis and cyphotic posture, highlighting their potential to improve functional movement capacity, reduce pain symptoms, and promote overall well-being. As reported in the study by Alkhathami et al.39, this tool is able to distinguish between individuals with and without LBP. The authors of this study finally state that it could be a useful test for physicians to evaluate mobility limitations and assess the quality of movement of an individual in people with low back pain. Moreover, other studies report the possible correlation between the FMS test and LBP for the evaluation of physical function40,41.
Software and hardware
The innovative tool for assessing and treating biomechanical alterations is a technological system designed for comprehensive movement analysis, consisting of a touchscreen interface and four high-velocity cameras specifically tailored for clinical settings. It addresses the limitations of traditional movement analysis tools by providing a user-friendly, portable, and cost-effective solution for clinicians.
Motion analysis system harnesses a powerful software suite to enable comprehensive movement analysis42, specifically tailored for clinical settings. This movement pattern assessment tool represents a groundbreaking innovation in clinical movement analysis, offering a user-friendly, portable, and affordable solution that has no previous versions. Unlike existing systems that rely on complex software and specialized hardware, the assessment tool described here streamlines the analysis process, making it accessible to a wider range of clinicians.
This suite comprises three key components: (i) Kinovea: Movement Analysis, (ii) Synology Surveillance Station: Efficient Video Management, and (iii) ApowerREC: Screen Capture and Annotation.
Kinovea, a widely employed video analysis software in biomechanics and movement science research. It allows joint angle assessment, empowering clinicians to precisely measure and analyze patients' movements. Its interface, coupled with advanced features for joint tracking, measurement, and visualization, makes it a suitable asset for delving into the intricacies of human movement. Whether in sports biomechanics, clinical assessments, or research settings, this video analysis software contributes to a precise assessment of joint angles and movement dynamics. Within the software suite, Kinovea is utilized for: (i) Joint Angle Assessment: Precisely measuring the angles of various joints during movement. (ii) Movement Pattern Analysis: Identifying specific movement patterns that contribute to pain or discomfort and monitoring treatment progress over time. (iii) Patient Feedback: Visually demonstrating movement patterns to patients to enhance their understanding and engagement in rehabilitation.
Synology Surveillance Station, a Video Management System (VMS), transforms Synology Network Attached Storage (NAS) devices into centralized monitoring solutions. Within the software suite, Surveillance Station plays a pivotal role in managing videos captured by the system's high-speed cameras. Its functionalities encompass: (i) Real-time Monitoring: Observing patients' movements during assessment sessions in real-time via video feeds. (ii) Video Playback and Analysis: Replaying recorded videos for a more thorough examination of movement patterns. (iii) User Management and Permissions: Controlling access to videos and analysis functionalities by authorized users.
ApowerREC serves to capture and annotate screen activity during analysis sessions. Its functionalities include: (i) Screen Recording: Capturing screen activity during movement analysis sessions at a frequency of 10 frames per second. (ii) Annotation Capabilities: Adding annotations, drawings, and comments to recorded videos to enhance communication and documentation. (iii) Recording Sharing: Easily sharing screen recordings with colleagues or patients. In combination, this software suite offers a potential solution for movement analysis in clinical settings.

Figure 2: System configuration for patient movement analysis. This figure shows the setup of the system, including the positioning of the cameras and the patient during movement analysis. Please click here to view a larger version of this figure.
The hardware is composed of the interactive monitor, shown in Figure 2. It served as the central control hub, allowing interaction and data acquisition during the movement assessment process. The four high-velocity cameras (Figure 2) were integral components of themotion capture and analysis system positioned to capture real-time dynamic movements. These cameras were equipped to record precise motion sequences, ensuring a thorough examination of the patient's motor function. Figure 3 shows the schematic representation of the setup for movement analysis.
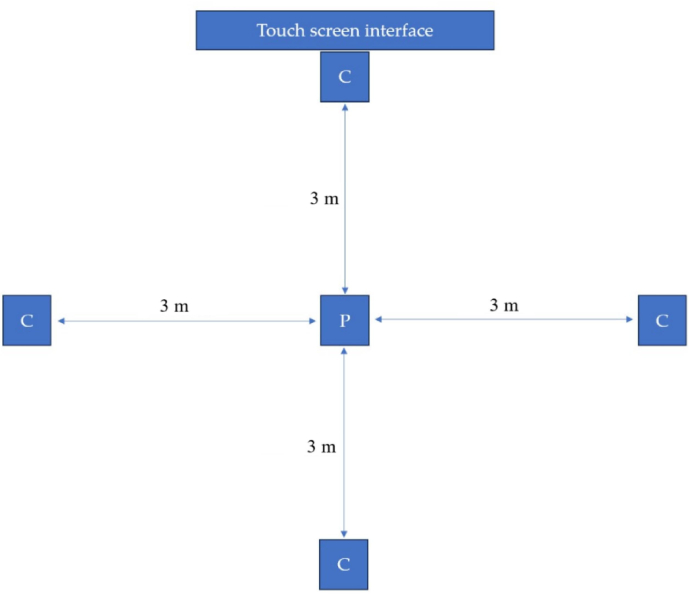
Figure 3: Schematic representation. A schematic representation of the setup highlighting the placement of high-velocity cameras (C) and the initial positioning of the patient (P). C: high-velocity camera; P: Starting position of the patient. Please click here to view a larger version of this figure.
The patient was positioned at the center, surrounded by four high-velocity cameras strategically placed at 3 m from the patient to capture a comprehensive view. The touchscreen interface served as the control hub for seamless interaction and real-time data acquisition during the assessment process. This configuration ensured thorough and detailed recording of dynamic movements, enabling a comprehensive analysis of motor function in clinical settings, particularly relevant for conditions such as LBP.
Assessment with functional movement screen (FMS)
Functional movement screen (FMS) is a system used to evaluate movement patterns and identify potential dysfunctions or limitations in physical performance43. It comprises a series of tests designed to assess fundamental movement patterns and asymmetries, aiding in injury prevention and performance optimization43. Although FMS is not a specific test for patients with LBP, this test is a validated tool for assessing an individual's functional movement capacity. While FMS tests were initially developed for athletes, their focus on fundamental movement patterns might be relevant for individuals with LBP where impaired movement patterns are closely linked with pain intensity and functional performance14,15,16. Figure 4 shows further details about the FMS test, completed with numeric and color final scores.
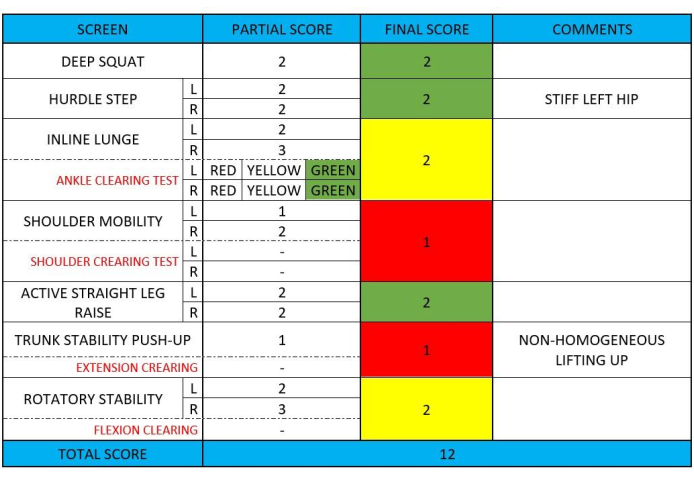
Figure 4: Example FMS test data collection. This figure presents an example of data collection during an FMS test, showing each single point of the test. Please click here to view a larger version of this figure.
As the FMS reported a green "traffic light" indicated that the exercises do not challenge the dysfunctional movement pattern. These exercises can be used safely during activities of daily living or training sessions. A yellow "traffic light" suggested that the movement pattern was correct, but it showed asymmetry between the two limbs. Therefore, caution is advised in programming. A red "traffic light" identified dysfunction in the execution of those motor patterns, and it is recommended to avoid such movements in programming because they needed for the training program43. The color code assigned to the final score had been holding significance for subsequent program planning (Table 2).
The assessment tool was used to assess the movement pattern precisely during the FMS test. It was performed in front of a Big-pad projecting real-time images from the cameras. Video analysis is considered fundamental to completing the investigation into movement quality and evaluating the motor execution strategy.
More in detail, the exercise assessed were the following:
Front squat, no hand: The first video-analyzed movement was a squat (two-legged movement) with the front positioning of the stick. This movement evaluated how the subject performed a squatting movement in a two-legged situation without the constraint of the "overhead" positioning that we had in the evaluation of the deep squat during the FMS evaluation part. The choice of this movement was introduced because this motor pattern can be traced back to various daily actions such as picking up an object from the ground, sitting down and getting up from a chair or sofa, etc., and it was therefore essential to learn and know how the subject carried out this movement in everyday life. In detail, the analysis of this movement involved the evaluation of two main investigation criteria: the lower limb control (on the frontal view) and the motor strategy used (on the lateral view). See Figure 5 for further details.
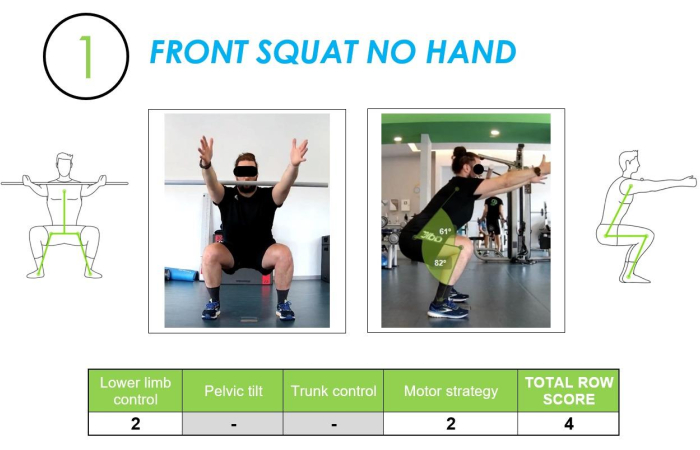
Figure 5: Front Squat (no hands) example assessed from frontal and lateral views, along with the corresponding row score. The figure shows a front squat (no hands) movement assessed from both frontal and lateral views, with the corresponding scoring of the movement quality. Please click here to view a larger version of this figure.
Lower limb control was assessed by tracing the axis between the center of the foot and the Anterior Superior Iliac Spine (ASIS) to identify and quantify the presence of a dynamic valgus in the knee joint. The analysis performed with the lateral camera images analyzed the flexion angles created on the knee and hip, determining whether the strategy used was correct and quantitatively sufficient.
Lower body motor control screen (LB-MCS): The second and the third tests were the single-leg squat (one-legged movement) for each side. See Figure 6 for further details. The analysis of this movement allowed us to assess the subject behavior in a single-legged situation. The control of a single-leg motor pattern has crucial implications in dynamic actions of activity of daily living, such as going up or down stairs, such as overcoming an obstacle, walking fast, or even running where there is a continuous alternation of single-legged positions.
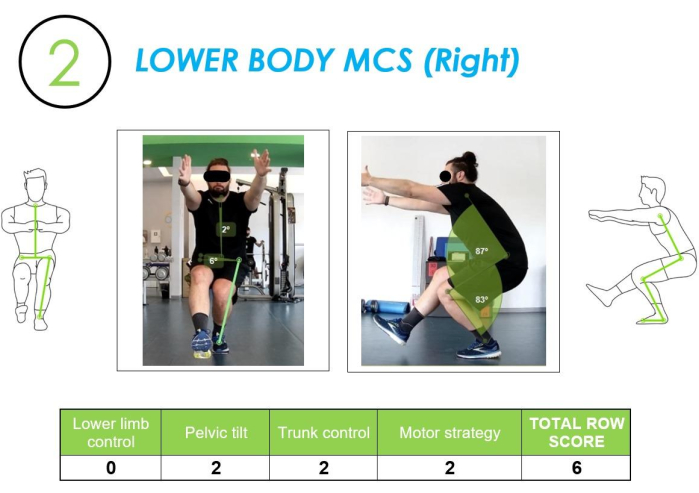
Figure 6: Lower body MCS analyzed from frontal and lateral views, along with the corresponding row score. The figure shows a lower body MCS movement assessed from both frontal and lateral views, with the corresponding scoring of the movement quality. Please click here to view a larger version of this figure.
In addition to the analysis of lower limb control and motor strategy, this test allowed evaluation of 1) the pelvis control through the analysis of the tilt angle that occurred between the ASIS with respect to the horizon and 2) trunk control by examining the angle of inclination between the midpoint of the ASIS and the jugular fossa.
Each movement underwent three separate assessments, and the one with the highest row score (see Table 1) was chosen for inclusion in the final report to calculate the total score. A frame was obtained from the video generated in both lateral and frontal views at the point of maximum descent.
Table 1: CameraLab test row score criteria. This table outlines the criteria used for scoring the movement assessments conducted with the assessment tool, detailing the parameters and scoring metrics applied. Please click here to download this Table.
The final page of the report provided information about the analysis and results. It also included advice on programming activities during the training program through re-learning motor pattern sessions or analytic sessions. In the training program, re-learning motor pattern sessions focused heavily on cognitive, associative, and automation phases through visual bio-feedback of correct dysfunctional movements, while in analytic sessions, more strenuous workloads were performed for general reinforcement, flexibility, and ROM recovery of functional movements.
Re-learning motor pattern sessions
Dysfunctional motor patterns were targeted by specific re-learning sessions by going through the three progressive phases of "motor learning"44.
Cognitive phase involves recognizing dysfunctional movement patterns, breaking down the complete movement into smaller components, and correcting these patterns through different forms of feedback provided by the system, including visual, spatial, and verbal feedback.
Associative phase: facilitating awareness of correct movement compared to the dysfunctional one, implementing self-correction. The operator progressively reduced the visual, verbal, and spatial feedback, leading the patient to learn the new correct motor pattern.
Automation phase: the patient performed the basic movements studied, analyzed, and corrected within the path without any type of visual, spatial, or verbal feedback, demanding self-correction in case of attitudes dysfunctional and carrying them out even in dual-tasking situations or with disruptive elements and/or functional overloads.
Analytic sessions
They were used to develop all those exercises most similar to conditional motor skills, such as strength, flexibility, and muscular and cardiovascular resistance. This type of session was also fundamental in improving the row and total score of the test as some movements analyzed within the test required a basic level of strength and flexibility on specific muscle groups such as the glutes, muscles belonging to the kinetic posterior chain such as the hamstrings or core muscles like the abdominals (transversus, rectus, obliques, etc.), latissimus dorsi, lumbar, adductors, etc. who needed conditioning through analytical exercises against resistance and with progressive overload.
The algorithm to determine which strategy to adopt (Table 2) considering re-learning motor pattern sessions and analytic sessions depended on which FMS movements graded red light and which video analysis criteria graded a row score ≤ 1.
Table 2: Algorithm to determine the strategy to adopt. This table shows the decision-making algorithm used to select intervention strategies based on FMS movement scores and video analysis criteria, directing the choice between re-learning motor pattern sessions and analytic sessions. Please click here to download this Table.
Once this process had been completed, it was possible to analyze, verify, and quantify the improvements that the patient had consolidated during the process through a follow-up test performed through the motion capture system.
Cases Presentation
Case 1 - Patient ID: AM
An 18-year-old Caucasian male, a professional student with a body mass index of 26.8 kg/m2 presented lumbar harmonic structured convex right scoliosis. The patient reported a chronic onset of LBP after prolonged sitting, with a medical history notable for severe scoliosis previously managed non-surgically (night corset for 4 years). The patient stated that the chronic pain had been present for over a year. His physical activity level was measured at 36 MET/week. Table 3 summarizes the patient's baseline characteristics.
During the initial examination, he reported minimal pain except when seated for an extended duration. Physical examination revealed that flexibility in the anterior and posterior chain was limited, as evidenced by restricted active mobility in the shoulders, thoracic shoulder girdle, and hips. The patient had a history of standard rehabilitation prior to presentation. Baseline assessment (T0) revealed that his NRS score was 4, SF-12 physical component summary (PCS) was 25.8, SF-12 mental component summary (MCS) was 46.2, RM was 4, and TSK was 36 (see Table 4 for further details). The evaluation with the motion capture system was implemented in the comprehensive patient assessment in order to characterize the patient's movement patterns and biomechanics. The assessment revealed an impairment in total FMS score (9/21), with impairments in shoulder mobility (score 1/3), active straight leg raise (score 1/3), trunk stability push-up (score 1/3), rotary stability (score 1/3), lower limb control (score 4/6), trunk control (score 3/4), and motor strategy (score 2/ 6). See Table 5 for further details.
Thus, the patient started standard rehabilitation intervention aimed at reducing pain, resolving inflammatory symptoms, and achieving strength recovery of specific muscles. More in detail, the patient performed a 12-session rehabilitation intervention, each lasting for 1 h, conducted over 3 days a week, focusing on a comprehensive approach. Therapy sessions included a warm-up to prepare the body for movement and decrease stiffness in the affected areas. Following the warm-up, the patient engaged in a series of targeted exercises designed to strengthen the core muscles, flexibility, and mobility exercises. Postural correction techniques were emphasized throughout the rehabilitation program to promote proper alignment of the spine and reduce strain on affected areas. The patient received education on ergonomic principles and learned strategies to maintain optimal posture during sitting, standing, and other activities of daily living.
A standard rehabilitation approach was implemented with biofeedback and motor control training using visual feedback from the system. This technology allowed the patient to observe their movement patterns in real time and make adjustments to improve posture and alignment. Through guided practice and repetition, the patient developed a greater awareness of their body mechanics and learned to perform movements more efficiently and effectively.
After the rehabilitation intervention (T1), consistent improvements were observed across all outcome measures, indicating positive progress in the patient's condition. The NRS score decreased to 2, while the SF-12-PCS increased to 41.0, and the MCS rose to 62.4. Additionally, the RM score decreased to 1, and the TSK score decreased to 25, reflecting improvements in pain levels, HR-QoL, disability, and fear of movement. Furthermore, the evaluation revealed notable enhancements in various movement parameters compared to the baseline. Specifically, improvements were observed in the deep squat, hurdle step, inline lunge, shoulder mobility, active straight leg raise, trunk stability push-up, rotary stability, lower limb control, pelvic tilt, trunk control, and motor strategy assessments. Table 5 shows further details about the scores for each evaluation test.
Case 2 - Patient ID: DB
A 38-year-old Caucasian male, a professional office employee, with a body mass index of 21.9 kg/m2, presented to our attention after microdiscectomy L4-L5. Before surgery, he reported pain 6/10 of NRS with irradiation up to the calf, paresthesia referred to the left thigh and leg, positive left Lasegue sign, and inability in common functional activity. The patient reported experiencing pain for eight months. Prior to surgery, he had pain therapy, acupuncture, massage therapy, and TENS.
Following a standard rehabilitation program, at sixty-four days post-surgery, the patient reported no pain, irradiation, or limitations in flexibility of the lower limb anterior and posterior chains. He reported right lower limb dominance in activities of daily living conditioned by fear of movement on the left side. The ability to stabilize the trunk with muscle was good in the analytic request of muscular activation (transversus abdominis, rectus abdominis, and internal and external obliques abdominals) but unable to maintain the stabilization during functional demands.
Baseline assessment revealed that his NRS score was 3, SF-12 PCS was 47.5, SF-12 MCS was 51.3, RM was 5, and TSK was 16 (see Table 4 for further details). The evaluation with the motion capture system was implemented in the comprehensive patient assessment in order to characterize the patient's movement patterns and biomechanics. The assessment revealed an impairment in total FMS score (10/21), with impairments in deep squat (score 1/3), shoulder mobility (score 2/3), active straight leg raise (score 0/3), pelvic tilt (score 3/4), and motor strategy (score 4/ 6). See Table 5 for further details. Thus, the patient performed standard rehabilitation intervention aimed at reducing pain, resolving inflammatory symptoms, recovering the full ROM and flexibility, and achieving strength recovery of specific muscles.
The patient performed 14 weeks of rehabilitation intervention, 3 sessions a week, each lasting for 1 h, the focus was on a comprehensive approach. Therapy sessions included a warm-up to prepare the body for movement and get better flexibility in the affected areas. Following the warm-up, the patient engaged in a series of targeted exercises designed to strengthen the core muscles and recovery exercises for active ROM. The restoration of the correct motor pattern was emphasized throughout the rehabilitation program to promote thoracic spine mobility, static and dynamic core exercises, gluteus exercises in static and dynamic versions, adding resistance too, squats, and lunges with a particular focus on the symmetry of the movements and the progressive removal of visual feedback. The patient performed drop jumps from boxes of increasing height, squat jump exercises training, and deceleration movements. The patient received education on principles and learned strategies to optimize the goal achieved and to reproduce the correct posture during all the activities of daily living.
A standard rehabilitation approach was implemented with biofeedback and motor control training using visual feedback from the system. This technology allowed the patient to observe their movement patterns in slow motion and make adjustments to improve posture, alignment, and motor patterns. Through guided practice, repetition, and progressively avoiding visual references, the patient developed a greater awareness of his own body mechanics and learned to perform movements more precisely, efficiently, and effectively.
After the rehabilitation intervention (T1), consistent improvements were observed across all outcome measures, indicating positive progress in the patient's condition. The NRS score decreased to 0, while the SF-12-PCS increased to 55.4, and the MCS rose to 54.7. Additionally, the RM score decreased to 1, and the TSK score decreased to 14, reflecting improvements in pain levels, HR-QoL, disability, and fear of movement. Furthermore, the evaluation revealed notable enhancements in various movement parameters compared to the baseline. Specifically, improvements were observed in the deep squat, shoulder mobility, active straight leg raise, pelvic tilt, and motor strategy assessments. Table 5 shows further details about the scores for each evaluation test.
Case 3 - patient ID: LB
A 33-year-old Caucasian male, a professional bartender with a body mass index of 24.8 kg/m2, presented to the attention of the clinic after surgery for lumbosacral spondylodiscitis. The patient had urgent right microdiscectomy L4-L5 surgery 40 days before spondylodiscitis surgery because he experienced a rapid loss of strength and lack of sensitivity in the right lower limb from the thigh to the foot over the course of 2 days.
At the conclusion of 20 days of hospitalization, during which the standard rehabilitation was administered, the patient reported pain in the lumbar spine, in the right lower limb, and sacral-iliac bilateral joints during postural shifts wearing corsets. The patient presented 2/5 of the Medical Research Council (MRC) Scale for all the muscles of the right lower limb. Core stability activation was poor both analytically and globally.
Baseline assessment (T0) revealed that his NRS score was 4, SF-12 PCS was 45.3, SF-12 MCS was 30.0, RM was 21, and TSK was 47 (see Table 4 for further details). The evaluation with the motion capture system was implemented in the comprehensive patient assessment to characterize patient's movement patterns and biomechanics. The assessment revealed an impairment in total FMS score (9/21), with impairments in Inline lunge (score 1/3), shoulder mobility (score 1/3), rotary stability (score 1/3), lower limb control (score 4/6), and motor strategy (score 2/ 6). See Table 5 for further details.
Thus, the patient continued the standard rehabilitation intervention lasting 12 weeks, 3 sessions a week, each session lasting for 1 h. Therapy sessions included a warm-up to prepare the body for active exercises, decrease stiffness in the affected areas, and activate the muscles involved in the rehabilitation session. Following the warm-up, the patient engaged in a series of targeted exercises designed to strengthen the core muscles, flexibility, and mobility exercise. Postural correction techniques were emphasized throughout the rehabilitation program to promote quadriceps, hamstrings, and gluteus muscles correct timing activation, single leg balance training, hip hinge strengthening using progressively bodyweight and ballast resistances, and static and dynamic core exercises. The patient performed squats, split squats, and lunges with a particular focus on the awareness of the alignment of his own body segments and the progressive removal of visual feedback and verbal correction by the therapist. The patient received education on ergonomic principles and learned strategies to maintain the right posture during sitting, standing, and other activities of daily living.
A standard rehabilitation approach was implemented with biofeedback and motor control training using visual feedback from the system. The implementation of this technology allowed the patient to observe his movement patterns, providing real-time feedback. This optimized adjustments to posture, alignment, and motor patterns. With guided practice and repetition, the patient enhanced his awareness of body mechanics and refined movement execution.
After the rehabilitation intervention (T1), consistent improvements were observed across all outcome measures, indicating positive progress in the patient's condition. The NRS score decreased to 1, while the SF-12-PCS increased to 53.9, and the MCS rose to 57.8. Additionally, the RM score decreased to 4, and the TSK score decreased to 39, reflecting improvements in pain levels, HR-QoL, disability, and fear of movement. Furthermore, the evaluation revealed notable enhancements in various movement parameters compared to the baseline. Specifically, improvements were observed in the inline lunge, shoulder mobility, rotary stability, lower limb control, and motor strategy assessments. Table 5 shows further details about the scores for each evaluation test.
Table 3: Population description. This table provides the demographic and clinical characteristics of the study population. Please click here to download this Table.
Table 4: Patient outcome. This table summarizes the outcomes for each patient involved in the study, including the changes observed after the final follow-up. Please click here to download this Table.
Table 5: Evaluation test results. This table details the results from the evaluation tests, presenting the performance metrics and movement scores for each assessed movement pattern. Please click here to download this Table.
Supplementary File 1: CARE structure and reporting guidelines. Please click here to download this File.
Discussion
In this study, we investigated the integration of the CameraLab system into the rehabilitation management of patients with LBP. The findings of this study suggested that this innovative digital solution provides valuable objective data on movement pattern analysis, enhancing the precision and effectiveness of clinical rehabilitation practice in musculoskeletal pain. LBP is a common and complex condition characterized by a multidimensional disability, including biomechanical, psychological, and social determinants 2,8,9,10,11,12. The approach reported in this case series allowed to target several factors characterizing LBP and addressing different domains as reported by the positive results in pain intensity, physical functioning, HR-QoL, and kinesiophobia.
More in detail, the findings of the case reports presented showed a consistent reduction in pain intensity by targeting specific movement patterns and biomechanical dysfunctions identified through the assessment tool. Interestingly, similar results were shown by Marich et al.45 in their study on musculoskeletal pain using a comparable assessment method. In their research, Marich et al.45 reported a potential link between movement patterns and functional limitations in individuals with chronic LBP. These findings highlighted the need for targeted interventions aimed at optimizing movement dysfunctions to reduce pain and improve functional outcomes in individuals with chronic LBP. Improvements in physical functioning and HR-QoL were shown in the three cases, as evidenced by increases in SF-12 PCS and MCS scores. Similarly, the study by Letafatkar et al.46 highlighted the effects of sensorimotor training protocols in improving proprioceptive system function, lumbar movement control, and HR-QoL for patients with chronic nonspecific LBP. The study underlined that a sensorimotor training program with innovative solutions led to consistent improvements in proprioception, lumbar movement control, and Hr-QoL46.
Kinesiophobia, is a common psychological barrier in individuals with LBP, often leading to avoidance behaviors and functional impairment47. The rehabilitation intervention with CameraLab successfully improved the fear of movement by providing objective feedback on movement patterns and biomechanics. By improving confidence in patients' ability to move safely and efficiently, the assessment tool might reduce kinesiophobia and promote active participation in rehabilitation activities. In this context, there has been a growing interest and investment in digital innovation and technological solutions within the field of rehabilitation25,26,48,49,50,51,52. This trend is driven by several factors, including advancements in sensor technology49,50, the increasing availability of portable devices25, and the growing recognition of the potential benefits of integrating digital tools into healthcare practices52. Digital solutions offer the promise of enhancing the delivery of rehabilitation services by providing objective data, improving patient engagement, and facilitating personalized treatment approaches53.
Several conditions might worsen LBP symptoms. In this context, Zaina et al.54 provided a comprehensive overview of the complexity of LBP in patients with and without scoliosis, highlighting that this condition significantly influences both physical and psychological aspects of health. This study highlights how advanced video-based motion analysis technology can accurately capture movement patterns and postural imbalances that contribute to LBP in a patient with scoliosis. This technology offers detailed insights into the biomechanical factors underlying pain that traditional assessment methods may underestimate. By enabling precise and objective assessment of motion, the approach described here provides a valuable tool for clinicians to develop more effective and personalized treatment plans, thereby improving patient outcomes.
Traditional methods of movement assessment, such as observational techniques or subjective clinical evaluations, are related to biases and difficulties in standardization20,27,28. In contrast, digital technologies, such as motion capture systems, inertial sensors, and computer vision algorithms, enable clinicians to capture and analyze movement data with a high degree of accuracy and reliability20. By quantifying movement parameters objectively, the assessment tool has shown the potential to identify biomechanical abnormalities, track progress over time, and tailor interventions to individual patient needs. Moreover, LBP can negatively impact disorders of kinesiological chains during movements, such as squats, through altered movement patterns. One study found that individuals with chronic LBP exhibit greater hip and knee range of motion relative to ankle ROM than those without LBP. These findings suggest that people with LBP overload their hip and knee joints more during squats, which may contribute to their condition55.
Moreover, Frontera et al.56 highlighted the importance of health policy and health services research in improving rehabilitation practices in real-life settings. The ability to accurately analyze and document movement patterns offers a significant advancement in rehabilitation. In support of this, our findings demonstrate that video analysis technology not only provides detailed biomechanical insights but also supports the development of more personalized and effective treatment strategies. This is in line with the study by Frontera et al.56, which aims to integrate research findings into clinical practice to bridge the gap between research and rehabilitation, ultimately strengthening the quality and accessibility of care for patients with LBP. By targeting specific movement patterns, CameraLab-guided interventions might enhance functional outcomes but might also have positive implications in the long-term, reducing recurrence rates of LBP and improving overall HR-QoL.
Besides these positive considerations, this study is not free from limitations. While CameraLab offers significant advantages in movement assessment, a specific initial setup and calibration of the system is necessary before rehabilitation starts. Moreover, the findings of the present manuscript are based on a small sample size, which aligns with the methodological framework of the case series. Although this approach allows for an in-depth analysis of each case, caution should be posed on the generalizability of the study results. Moreover, different cases with heterogeneous causes of LBP were assessed in this case series. However, this study might offer preliminary insights about an innovative technology that might be further studied in larger cohort studies with homogeneous samples. Lastly, the cost and availability of the technology may limit its widespread adoption in clinical settings. On the other hand, it should be noted that this technology might be one of the most inexpensive and cost-effective in rehabilitation settings compared to similar movement pattern analysis systems. In accordance with other similar movement pattern analysis systems, there might be a risk of bias in inter-operator repeatability, landmark identification, or selection of the maximum descent point57. To address these limitations, we ensure that all personnel involved in the technique are adequately trained and experienced. Future research should aim to validate the effectiveness of this assessment tool further in larger patient populations and compare its outcomes with traditional rehabilitation approaches.
In conclusion, our study suggests that the CameraLab system could have a role in the rehabilitation management of patients with LBP. By providing objective data on movement patterns and facilitating targeted interventions, the assessment tool has the potential to improve outcomes and implement clinical practice. Further research is needed to fully understand its implications and optimize its integration into routine care.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
This study is part of the project NODES which has received funding from the MUR - M4C2 1.5 of PNRR with grant agreement no. ECS00000036.
Materials
| Name | Company | Catalog Number | Comments |
| ApowerREC | Apowersoft | https://www.apowersoft.com/record-all-screen | This screen recorder serves to capture and annotate screen activity during analysis sessions |
| Functional Movement Screen kit | Functional Movement Systems Inc., Chatham, VA | N/A | Funtional Movement Screen kit consisting of a two-inch by six-inch board, one four-foot-long dowel, two short dowels, and an elastic cord, is used to administer the FMS test. |
| Hikvision Cameras IP POE DOME | Hikvision | DS-2CD1623G0-IZ | The cameras are equipped to record precise motion sequences and to capture dynamic movements with exceptional speed and detail. |
| Kinovea | Kinovea | Version 0.9.5 | Kinovea is a video annotation tool designed for sport analysis. It features utilities to capture, slow down, compare, annotate and measure motion in videos. |
| Sharp Big Pad (PN-85 TH1) | Sharp Corporation | PN-85 TH1 | The PN-85TH1 interactive BIG PAD monitor combines "4K reading" and the "Pen-on-Paper" user experience with the high precision of InGlass touch technology. Includes whiteboard and wireless capabilities to further enhance the customer experience |
| Synology Surveillance Station | Synology | N/A | Robust and versatile Video Management System (VMS) designed to turn Synology Network Attached Storage (NAS) devices into centralized surveillance solutions |
References
- Tanaka, Y., et al. Muscle strength rather than appendicular skeletal muscle mass might affect spinal sagittal alignment, low back pain, and health-related quality of life. Sci Rep. 13 (1), 9894 (2023).
- de Sire, A., et al. Pharmacological treatment for acute traumatic musculoskeletal pain in athletes. Medicina. 57 (11), 1208 (2021).
- GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990-2020, its attributable risk factors, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 5 (6), e316-e329 (2023).
- Bailey, J. F., et al. Digital care for chronic musculoskeletal pain: 10,000 participant longitudinal cohort study. J Med Internet Res. 22 (5), e18250 (2020).
- Priebe, J. A., et al. Digital treatment of back pain versus standard of care: the cluster-randomized controlled trial, Rise-uP. J Pain Res. 13, 1823-1838 (2020).
- Chehade, M. J., et al. Innovations to improve access to musculoskeletal care. Best Pract Res Clin Rheumatol. 34 (5), 101559 (2020).
- GBD Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 396 (10258), 1204-1222 (2020).
- Lippi, L., et al. Multidimensional effectiveness of botulinum toxin in neuropathic pain: a systematic review of randomized clinical trials. Toxins. 14 (5), 308 (2022).
- de Sire, A., et al. Ultrasound-guided platelet-rich-plasma injections for reducing sacroiliac joint pain: A paradigmatic case report and literature review. J Back Musculoskelet Rehabil. 35 (5), 977-982 (2022).
- de Sire, A., et al. Dynamic spinal orthoses self-reported effects in patients with back pain due to vertebral fragility fractures: A multi-center prospective cohort study. J Back Musculoskelet Rehabil. 37 (4), 929-941 (2023).
- de Sire, A., et al. Percutaneous electrical nerve stimulation (Pens) as a rehabilitation approach for reducing mixed chronic pain in patients with musculoskeletal disorders. Appl Sci. 11 (9), 4257 (2021).
- Marotta, N., et al. Impact of yoga asanas on flexion and relaxation phenomenon in women with chronic low back pain: Prophet model prospective study. J Orthop Res. 42 (7), 1420-1427 (2024).
- . WHO Guideline for Non-Surgical Management of Chronic Primary Low Back Pain in Adults in Primary and Community Care Settings Available from: https://www.who.int/publications/i/item/9789240081789 (2023)
- Urban, J. P., Fairbank, J. C. Current perspectives on the role of biomechanical loading and genetics in development of disc degeneration and low back pain; a narrative review. J Biomech. 102, 109573 (2020).
- Wu, Z., et al. Asymmetric biomechanical properties of the paravertebral muscle in elderly patients with unilateral chronic low back pain: a preliminary study. Front Bioeng Biotechnol. 10, 814099 (2022).
- Wernli, K., et al. Does movement change when low back pain changes? A systematic review. J Orthop Sports Phys Ther. 50 (12), 664-670 (2020).
- Hira, K., et al. Relationship of sagittal spinal alignment with low back pain and physical performance in the general population. Sci Rep. 11 (1), 20604 (2021).
- Hlaing, S. S., Puntumetakul, R., Khine, E. E., Boucaut, R. Effects of core stabilization exercise and strengthening exercise on proprioception, balance, muscle thickness and pain related outcomes in patients with subacute nonspecific low back pain: a randomized controlled trial. BMC Musculoskelet Disord. 22 (1), 998 (2021).
- Yasuda, T., Jaotawipart, S., Kuruma, H. Effects of thoracic spine self-mobilization on patients with low back pain and lumbar hypermobility: A randomized controlled trial. Prog Rehabil Med. 8, 20230022 (2023).
- van Dijk, M. J., et al. Assessment instruments of movement quality in patients with nonspecific low back pain: A systematic review and selection of instruments. Gait Posture. 76, 346-357 (2020).
- Hlaing, S. S., Puntumetakul, R., Wanpen, S., Boucaut, R. Balance control in patients with subacute nonspecific low back pain, with and without lumbar instability: a cross-sectional study. J Pain Res. 13, 795-803 (2020).
- Lippi, L., et al. Effects of blood flow restriction on spine postural control using a robotic platform: A pilot randomized cross-over study. J Back Musculoskelet Rehabil. 36 (6), 1447-1459 (2023).
- Sipko, T., Glibowski, E., Kuczyński, M. Acute effects of proprioceptive neuromuscular facilitation exercises on the postural strategy in patients with chronic low back pain. Complement Ther Clin Pract. 44, 101439 (2021).
- Desmons, M., Theberge, M., Mercier, C., Massé-Alarie, H. Contribution of neural circuits tested by transcranial magnetic stimulation in corticomotor control of low back muscle: a systematic review. Front Neurosci. 17, 1180816 (2023).
- Lippi, L., et al. System for tracking and evaluating performance (Step-App®): validation and clinical application of a mobile telemonitoring system in patients with knee and hip total arthroplasty. A prospective cohort study. Eur J Phys Rehabil Med. 60 (2), 349-360 (2024).
- de Sire, A., et al. Myths and truths on biophysics-based approach in rehabilitation of musculoskeletal disorders. Ther Adv in Musculoskelet Dis. 15, 1759720X231183867 (2023).
- Garg, A., Pathak, H., Churyukanov, M. V., Uppin, R. B., Slobodin, T. M. Low back pain: critical assessment of various scales. Eur Spine J. 29 (3), 503-518 (2020).
- Streicher, H. New concepts in back class training? Effects of a therapeutical back class training focussing on proprioceptive-coordinative skills. Deutsche Zeitschrift fur Sportmedizin. 56 (4), 100-105 (2005).
- Hamacher, D., Hamacher, D., Herold, F., Schega, L. Are there differences in the dual-task walking variability of minimum toe clearance in chronic low back pain patients and healthy controls. Gait Posture. 49, 97-101 (2016).
- van Hoof, W., Volkaerts, K., O'Sullivan, K., Verschueren, S., Dankaerts, W. Comparing lower lumbar kinematics in cyclists with low back pain (flexion pattern) versus asymptomatic controls-field study using a wireless posture monitoring system. Man Ther. 17 (4), 312-317 (2012).
- AlAteeq, M., Alseraihi, A. A., Alhussaini, A. A., Binhasan, S. A., Ahmari, E. A. Plain lumbosacral X-rays for low back pain: Findings correlate with clinical presentation in primary care settings. J Family Med Prim Care. 9 (12), 6115-6120 (2020).
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 310 (20), 2191-2194 (2013).
- Cook, G., Burton, L., Hoogenboom, B. J., Voight, M. Functional movement screening: the use of fundamental movements as an assessment of function-part 1. Int J Sports Phys Ther. 9 (3), 396-409 (2014).
- Cook, G., Burton, L., Hoogenboom, B. J., Voight, M. Functional movement screening: the use of fundamental movements as an assessment of function-part 2. Int J Sports Phys Ther. 9 (4), 549-563 (2014).
- Bijur, P. E., Latimer, C. T., Gallagher, E. J. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 10 (4), 390-392 (2003).
- Luo, X., et al. Reliability, responsiveness of the short form 12-item survey (SF-12) in patients with back pain. Spine (Phila Pa 1976). 28 (15), 1739-1745 (2003).
- Küçükdeveci, A. A., Tennant, A., Elhan, A. H., Niyazoglu, H. Validation of the Turkish version of the Roland-Morris Disability Questionnaire for use in low back pain. Spine (Phila Pa). 26 (24), 2738-2743 (2001).
- Monticone, M., Ambrosini, E., Rocca, B., Foti, C., Ferrante, S. Responsiveness of the Tampa Scale of Kinesiophobia in Italian subjects with chronic low back pain undergoing motor and cognitive rehabilitation. Eur Spine J. 25 (9), 2882-2888 (2016).
- Alkhathami, K., Alshehre, Y., Wang-Price, S., Brizzolara, K. Reliability and validity of the Functional Movement Screen™ with a modified scoring system for young adults with low back pain. Int J Sports Phys Ther. 16 (3), 620-627 (2021).
- Alkhathami, K. M., Alqahtani, B. Comparing the scores of the Functional Movement Screen™ in individuals with low back pain versus healthy individuals: A systematic review and meta-analysis. Int J Sports Phys Ther. 19 (7), 834-848 (2024).
- Ko, M. J., Noh, K. H., Kang, M. H., Oh, J. S. Differences in performance on the functional movement screen between chronic low back pain patients and healthy control subjects. J Phys Ther Sci. 28 (7), 2094-2096 (2016).
- Puig-Diví, A., et al. Validity and reliability of the Kinovea program in obtaining angles and distances using coordinates in 4 perspectives. PLoS One. 14 (6), e0216448 (2019).
- Schneiders, A. G., Davidsson, &. #. 1. 9. 7. ;., Hörman, E., Sullivan, S. J. Functional movement screenTM normative values in a young, active population. Int J Sports Phys Ther. 6 (2), 75 (2011).
- Cuenca-Martínez, F., Suso-Martí, L., León-Hernández, J. V., La Touche, R. The role of movement representation techniques in the motor learning process: A neurophysiological hypothesis and a narrative review. Brain Sci. 10 (1), 27 (2020).
- Marich, A. V., Hwang, C. T., Sorensen, C. J., Van Dillen, L. R. Examination of the Lumbar movement pattern during a clinical test and a functional activity test in people with and without low back pain. PM R. 12 (2), 140-146 (2020).
- Letafatkar, A., Nazarzadeh, M., Hadadnezhad, M., Farivar, N. The efficacy of a HUBER exercise system mediated sensorimotor training protocol on proprioceptive system, lumbar movement control and quality of life in patients with chronic non-specific low back pain. J Back Musculoskelet Rehabil. 30 (4), 767-778 (2017).
- Luque-Suarez, A., Martinez-Calderon, J., Falla, D. Role of kinesiophobia on pain, disability and quality of life in people suffering from chronic musculoskeletal pain: a systematic review. Br J Sports Med. 53 (9), 554-559 (2019).
- Nascimben, M., Lippi, L., Fusco, N., Invernizzi, M., Rimondini, L. A software suite for limb volume analysis applicable in clinical settings: upper limb quantification. Front Bioeng Biotechnol. 10, 863689 (2022).
- Nascimben, M., et al. Technical aspects and validation of custom digital algorithms for hand volumetry. Technol Health Care. 31 (5), 1835-1854 (2023).
- Invernizzi, M., et al. Integrating augmented reality tools in breast cancer related lymphedema prognostication and diagnosis. J Vis Exp. (156), e60093 (2020).
- de Sire, A., et al. Three-dimensional laser scanning as a reliable and reproducible diagnostic tool in breast cancer related lymphedema rehabilitation: a proof-of-principle study. Eur Rev Med Pharmacol Sci. 24 (8), 4476-4485 (2020).
- Lippi, L., et al. Technological advances and digital solutions to improve quality of life in older adults with chronic obstructive pulmonary disease: a systematic review. Aging Clin Exp Res. 35 (5), 953-968 (2023).
- Marotta, N., et al. Integrating virtual reality and exergaming in cognitive rehabilitation of patients with Parkinson disease: a systematic review of randomized controlled trials. Eur J Phys Rehabil Med. 58 (6), 818-826 (2022).
- Zaina, F., et al. Measuring quality of life in adults with scoliosis: A cross-sectional study comparing SRS-22 and ISYQOL questionnaires. J Clin Med. 12 (15), 5071 (2023).
- Zawadka, M., et al. Altered squat movement pattern in patients with chronic low back pain. Ann Agric Environ Med. 28 (1), 158-162 (2021).
- Frontera, W. R., et al. Relevance and use of health policy, health systems and health services research for strengthening rehabilitation in real-life settings: methodological considerations. Eur J Phys Rehabil Med. 60 (1), 154-163 (2024).
- Dingenen, B., et al. Can two-dimensional video analysis during single-leg drop vertical jumps help identify non-contact knee injury risk? A one-year prospective study. Clin Biomech (Bristol, Avon). 30 (8), 781-787 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved