Creating a Winogradsky Column: A Method to Enrich the Microbial Species in a Sediment Sample
Genel Bakış
Source: Elizabeth Suter1, Christopher Corbo1, Jonathan Blaize1
1 Department of Biological Sciences, Wagner College, 1 Campus Road, Staten Island NY, 10301
The Winogradsky column is a miniature, enclosed ecosystem used for enriching sediment microbial communities, especially those involved in sulfur cycling. The column was first used by Sergei Winogradsky in the 1880s and has since been applied in the study of many diverse microorganisms involved in biogeochemistry, such as photosynthesizers, sulfur oxidizers, sulfate reducers, methanogens, iron oxidizers, nitrogen cyclers, and more (1,2).
The majority of microorganisms on Earth are considered unculturable, meaning that they cannot be isolated in a test tube or on a petri dish (3). This is due to many factors, including that microorganisms depend on others for certain metabolic products. The conditions in a Winogradsky column closely mimic a microorganism's natural habitat, including their interactions with other organisms, and allows for them to be grown in a lab. Therefor, this technique permits scientists to study these organisms and understand how they are important to Earth's biogeochemical cycles without having to grow them in isolation.
Earth's environments are full of microorganisms which thrive in all types of habitats, such as soils, ocean water, clouds, and deep-sea sediments. In all habitats, microorganisms depend on each other. As a microorganism grows, it consumes particular substrates, including carbon-rich fuels like sugars as well as nutrients, vitamins, and respiratory gases like oxygen. When these important resources run out, different microorganisms with different metabolic needs can then bloom and thrive. For example, in the Winogradsky column, microbes first consume the added organic material while depleting the oxygen in the bottom layers of the column. Once the oxygen is used up, anaerobic organisms can then take over and consume different organic materials. This consecutive development of different microbial communities over time is called succession (4). Microbial succession is important in a Winogradsky column, where microbial activity changes the chemistry of the sediment, which then affects activity of other microbes and so on. Many microorganisms in soils and sediments also live along gradients, which are transitional zones between two different types of habitats based on the concentrations of substrates (5). At the correct spot in the gradient, a microbe can receive optimal amounts of different substrates. As a Winogradsky column develops, it begins to mimic these natural gradients, particularly in oxygen and sulfide (Fig. 1).
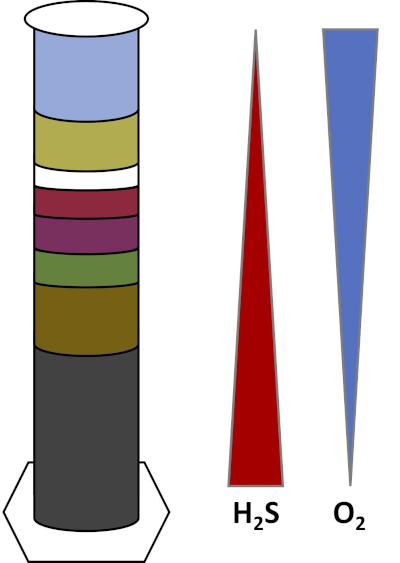
Figure 1: A representation of the oxygen (O2) and sulfide (H2S) gradients that develop in a Winogradsky column.
In a Winogradsky column, mud and water from a pond or wetland are mixed in a transparent column and allowed to incubate, typically in the light. Additional substrates are added to the column to give the community sources of carbon, usually in the form of cellulose, and sulfur. Photosynthesizers typically start to grow in the top layers of the sediment. These photosynthetic microorganisms are largely composed of cyanobacteria, which produce oxygen and appear as a green or red-brown layer (Fig. 2, Table 1). While photosynthesis produces oxygen, oxygen is not very soluble in water and it decreases below this layer (Fig. 1). This creates a gradient of oxygen, ranging from high concentrations of oxygen in the top layers to zero oxygen in the bottom layers. The oxygenated layer is called the aerobic layer and the layer without oxygen is called the anaerobic layer.
In the anaerobic layer, many different microbial communities can proliferate depending on the type and amount of substrates that are available, the source of the initial microbes, and the porosity of the sediment. At the bottom of the column, organisms that anaerobically break down organic matter can thrive. Microbial fermentation produces organic acids from the breakdown of cellulose. These organic acids can then be used by sulfate reducers, which oxidize those organics using sulfate, and produce sulfide as a byproduct. The activity of sulfate reducers is indicated if the sediment turns black, because iron and sulfide react to form black iron-sulfide minerals (Fig. 2, Table 1). The sulfide also diffuses upward, creating another gradient in which sulfide concentrations are high in the bottom of the column and low in the top of the column (Fig. 1).
Near the middle of the column, sulfur oxidizers take advantage of the supply of oxygen from above and sulfide from below. With the right amount of light, photosynthetic sulfur oxidizers can develop in these layers. These organisms are known as green and purple sulfur bacteria, and often appear as green, purple, or purple-red filaments and blotches (Fig. 2, Table 1). Green sulfur bacteria have a higher tolerance for sulfide and usually develop in the layer directly below purple sulfur bacteria. Above the purple sulfur bacteria, purple nonsulfur bacteria may also develop. These organisms photosynthesize using organic acids as electron donors instead of sulfide and often appear as a red, purple, orange, or brown layer. Nonphotosynthetic sulfur oxidizers can develop above the purple nonsulfur bacteria, and these usually appear as white filaments (Fig. 2, Table 1). Additionally, bubbles may also form in the Winogradsky column. Bubbles in the aerobic layers indicates the production of oxygen by the cyanobacteria. Bubbles in the anaerobic layers are likely due to the activity of methanogens, organisms which anaerobically break down organic matter and form methane as a byproduct.
| Position in Column | Functional group | Organism Examples | Visual Indicator |
| Top | Photosynthesizers | Cyanobacteria | Green or reddish-brown layer. Sometimes bubbles of oxygen. |
| Nonphotosynthetic sulfur oxidizers | Beggiatoa, Thiobacilus | White layer. | |
| Purple nonsulfur bacteria | Rhodomicrobium, Rhodospirilum, Rhodopseuodmonas | Red, purple, orange, or brown layer. | |
| Purple sulfur bacteria | Chromatium | Purple, or purple-red layer. | |
| Green sulfur bacteria | Chlorobium | Green layer. | |
| Sulfate Reducing Bacteria | Desulfovibrio, Desulfotomaculum, Desulfobacter, Desulfuromonas | Black layer. | |
| Bottom | Methanogens | Methanococcus, Methanosarcina | Sometimes bubbles of methane. |
Table 1: The main groups of bacteria that may appear in a classical Winogradsky column, from top to bottom. Examples of organisms from each group are given, and the visual indicators of each layer of organisms are listed. Based on Perry et al. (2002) and Rogan et al. (2005).
Prosedür
1. Set-up
- To set up a Winogradsky column, you will need some basic supplies:
- A shovel, bucket, and bottle to collect the samples in the field
- A vertical, transparent vessel, such as a graduated cylinder or plastic water bottle of about 1L
- Plastic wrap and rubber bands
- large mixing bowls and large spoon to stir
- A sulfur source (egg yolk or calcium sulfate)
- A source of organic carbon (cellulose, in the form of shredded newspaper
Sonuçlar
In this experiment, water and sediment were collected from a freshwater habitat. Two Winogradsky columns were constructed and allowed to develop: a classical Winogradsky column incubated in the light at room temperature (Fig. 2A) and a Winogradsky column incubated in the dark at room temperature (Fig. 2B).

Figure 2B: A photo of classical Winogradsky column (left), incubated at room tempe
Başvuru ve Özet
The Winogradsky column is an example of an interdependent microbial ecosystem. After mixing mud, water, and additional carbon and sulfur substrates in a vertical column, the stratified ecosystem should stabilize into separate, stable zones over several weeks. These zones are occupied by different microorganisms which flourish at a particular spot along the gradient between the sulfide-rich sediment in the bottom and the oxygen-rich sediment at the top. By manipulating the conditions and substrates within the Winogradsky
Referanslar
- Zavarzin G. (2006). Winogradsky and modern microbiology. Microbiology 75(6): 501-511. doi: 10.1134/s0026261706050018
- Esteban DJ, Hysa B, and Bartow-McKenney C (2015). Temporal and Spatial Distribution of the Microbial Community of Winogradsky Columns. PLoS ONE 10(8): e0134588. doi:10.1371/journal.pone.0134588
- Lloyd KG, Steen AD, Ladau J, Yin J, and Crosby L. (2018). Phylogenetically novel uncultured microbial cells dominate Earth microbiomes. mSystems 3(5): e00055-18. doi:10.1128/mSystems.00055-18
- Anderson DC, and Hairston RV (1999). The Winogradsky Column & Biofilms: Models for Teaching Nutrient Cycling & Succession in an Ecosystem. The American Biology Teacher, 61(6): 453-459. doi: 10.2307/4450728
- Dang H, Klotz MG, Lovell CR and Sievert SM (2019) Editorial: The Responses of Marine Microorganisms, Communities and Ecofunctions to Environmental Gradients. Frontiers in Microbiology 10(115). doi: 10.3389/fmicb.2019.00115
- Stomp M, Huisman J, Stal LJ, and Matthijs HCP. (2007) Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. ISME Journal. 1(4): 271-282. doi:10.1038/ismej.2007.59
- Perry JJ, Staley JT, and Lory S. (2002) Microbial Life, First Edition, published by Sinauer Associates
- Rogan B, Lemke M, Levandowsky M, and Gorrel T. (2005) Exploring the Sulfur Nutrient Cycle Using the Winogradsky Column. The American Biology Teacher, 67(6): 348-356. doi: 10.2307/4451860
Atla...
Bu koleksiyondaki videolar:

Now Playing
Creating a Winogradsky Column: A Method to Enrich the Microbial Species in a Sediment Sample
Microbiology
130.2K Görüntüleme Sayısı

Serial Dilutions and Plating: Microbial Enumeration
Microbiology
317.4K Görüntüleme Sayısı

Enrichment Cultures: Culturing Aerobic and Anaerobic Microbes on Selective and Differential Medias
Microbiology
132.4K Görüntüleme Sayısı

Pure Cultures and Streak Plating: Isolation of Single Bacterial Colonies from a Mixed Sample
Microbiology
166.6K Görüntüleme Sayısı

16S rRNA Sequencing: A PCR-based Technique to Identify Bacterial Species
Microbiology
189.8K Görüntüleme Sayısı

Growth Curves: Generating Growth Curves Using Colony Forming Units and Optical Density Measurements
Microbiology
298.2K Görüntüleme Sayısı

Antibiotic Susceptibility Testing: Epsilometer Tests to Determine MIC Values of Two Antibiotics and Evaluate Antibiotic Synergy
Microbiology
94.2K Görüntüleme Sayısı

Microscopy and Staining: Gram, Capsule, and Endospore Staining
Microbiology
364.3K Görüntüleme Sayısı

Plaque Assay: A Method to Determine Viral Titer as Plaque Forming Units (PFU)
Microbiology
186.7K Görüntüleme Sayısı

Transformation of E. coli Cells Using an Adapted Calcium Chloride Procedure
Microbiology
87.2K Görüntüleme Sayısı

Conjugation: A Method to Transfer Ampicillin Resistance from Donor to Recipient E. coli
Microbiology
38.5K Görüntüleme Sayısı

Phage Transduction: A Method to Transfer Ampicillin Resistance from Donor to Recipient E. coli
Microbiology
29.3K Görüntüleme Sayısı
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır
