Création d'une colonne de Winogradsky : une méthode pour enrichir les espèces microbiennes dans un échantillon de sédiments.
Vue d'ensemble
Source: Elizabeth Suter1, Christopher Corbo1, Jonathan Blaize1
1 Département des sciences biologiques, Wagner College, 1 Campus Road, Staten Island NY, 10301
La colonne Winogradsky est un écosystème miniature et clos utilisé pour enrichir les communautés microbiennes des sédiments, en particulier celles qui participent au cycle du soufre. La colonne a été utilisée pour la première fois par Sergueï Winogradsky dans les années 1880 et a depuis été appliquée dans l'étude de nombreux micro-organismes divers impliqués dans la biogéochimie, tels que les photosynthétiseurs, les oxydants de soufre, les réducteurs de sulfate, les méthanogènes, les oxydants de fer, l'azote cyclistes, et plus (1,2).
La majorité des micro-organismes sur Terre sont considérés comme inexplicables,ce qui signifie qu'ils ne peuvent pas être isolés dans un tube à essai ou sur un plat de Pétri (3). Cela est dû à de nombreux facteurs, y compris que les micro-organismes dépendent d'autres pour certains produits métaboliques. Les conditions d'une colonne Winogradsky imitent étroitement l'habitat naturel d'un micro-organisme, y compris leurs interactions avec d'autres organismes, et permettent de les cultiver en laboratoire. Par la présente, cette technique permet aux scientifiques d'étudier ces organismes et de comprendre à quel point ils sont importants pour les cycles biogéochimiques de la Terre sans avoir à les cultiver isolément.
Les environnements de la Terre sont pleins de micro-organismes qui prospèrent dans tous les types d'habitats,tels que les sols, l'eau de mer, les nuages et les sédiments des eaux profondes. Dans tous les habitats, les micro-organismes dépendent les uns des autres. Comme un micro-organisme se développe, il consomme des substratsparticuliers , y compris les combustibles riches en carbone comme les sucres ainsi que les nutriments, vitamines et gaz respiratoires comme l'oxygène. Lorsque ces ressources importantes s'épuisent, différents micro-organismes ayant des besoins métaboliques différents peuvent alors fleurir et prospérer. Par exemple, dans la colonne Winogradsky, les microbes consomment d'abord la matière organique ajoutée tout en appauvrissant l'oxygène dans les couches inférieures de la colonne. Une fois que l'oxygène est utilisé, les organismes anaérobies peuvent alors prendre le relais et consommer différentes matières organiques. Ce développement consécutif de différentes communautés microbiennes au fil du temps s'appelle la succession (4). La succession microbienne est importante dans une colonne Winogradsky, où l'activité microbienne modifie la chimie des sédiments, ce qui affecte ensuite l'activité d'autres microbes et ainsi de suite. De nombreux micro-organismes dans les sols et les sédiments vivent également le long des gradients,qui sont des zones transitoires entre deux types différents d'habitats en fonction des concentrations de substrats (5). Au bon endroit dans le gradient, un microbe peut recevoir des quantités optimales de différents substrats. Au fur et à mesure qu'une colonne Winogradsky se développe, elle commence à imiter ces gradients naturels, en particulier dans l'oxygène et le sulfure (fig. 1).
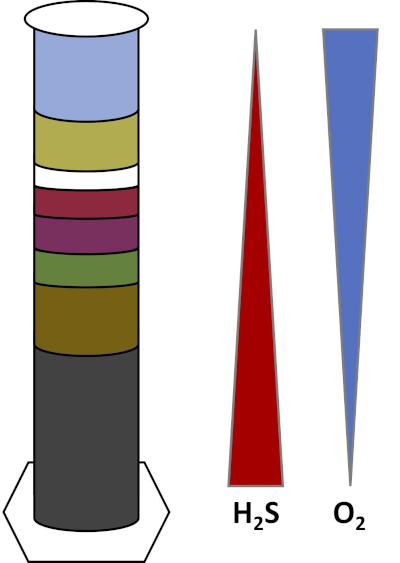
Figure 1 : Représentation des gradients d'oxygène (O2) et de sulfure (H2S) qui se développent dans une colonne Winogradsky.
Dans une colonne Winogradsky, la boue et l'eau d'un étang ou d'une zone humide sont mélangées dans une colonne transparente et autorisées à incuber, généralement dans la lumière. Des substrats supplémentaires sont ajoutés à la colonne pour donner à la communauté des sources de carbone, généralement sous forme de cellulose, et de soufre. Les photosynthétiseurs commencent généralement à pousser dans les couches supérieures des sédiments. Ces micro-organismes photosynthétiques sont en grande partie composés de cyanobactéries,qui produisent de l'oxygène et apparaissent sous forme de couche verte ou rouge-brun (fig. 2, tableau 1). Bien que la photosynthèse produise de l'oxygène, l'oxygène n'est pas très soluble dans l'eau et diminue en dessous de cette couche (fig. 1). Cela crée un gradient d'oxygène, allant de fortes concentrations d'oxygène dans les couches supérieures à zéro oxygène dans les couches inférieures. La couche oxygénée est appelée la couche aérobie et la couche sans oxygène est appelée la couche anaérobie.
Dans la couche anaérobie, de nombreuses communautés microbiennes différentes peuvent proliférer selon le type et la quantité de substrats disponibles, la source des microbes initiaux et la porosité des sédiments. Au bas de la colonne, les organismes qui décomposent anaérobiement la matière organique peuvent prospérer. La fermentation microbienne produit des acides organiques à partir de la dégradation de la cellulose. Ces acides organiques peuvent ensuite être utilisés par les réducteurs de sulfate,qui oxydent ces matières organiques à l'aide de sulfate, et produisent du sulfure comme sous-produit. L'activité des réducteurs de sulfate est indiquée si les sédiments deviennent noirs, car le fer et le sulfure réagissent pour former des minéraux noirs sulfureux de fer (fig. 2, tableau 1). Le sulfure se diffuse également vers le haut, créant un autre gradient dans lequel les concentrations de sulfure sont élevées dans le bas de la colonne et basses dans le haut de la colonne (fig. 1).
Près du milieu de la colonne, les oxydants de soufre profitent de l'approvisionnement en oxygène d'en haut et de sulfure d'en bas. Avec la bonne quantité de lumière, des oxydants de soufre photosynthétiques peuvent se développer dans ces couches. Ces organismes sont connus sous le nom de bactéries de soufre vert et violet,et apparaissent souvent sous forme de filaments et de taches vert, violet ou rouge pourpre (fig. 2, tableau 1). Les bactéries de soufre vert ont une plus grande tolérance pour le sulfure et se développent habituellement dans la couche directement au-dessous des bactéries de soufre pourpre. Au-dessus des bactéries de soufre pourpre, les bactéries nonsulfures pourpres peuvent également se développer. Ces organismes photosynthétisent à l'aide d'acides organiques comme donneurs d'électrons au lieu de sulfure et apparaissent souvent comme une couche rouge, pourpre, orange ou brune. Les oxydants de soufre non photosynthétiques peuvent se développer au-dessus des bactéries non sulfureuses violettes, et ceux-ci apparaissent habituellement sous forme de filaments blancs (Fig. 2, tableau 1). En outre, des bulles peuvent également se former dans la colonne Winogradsky. Les bulles dans les couches aérobies indiquent la production d'oxygène par les cyanobactéries. Les bulles dans les couches anaérobies sont probablement dues à l'activité des méthanogènes,des organismes qui décomposent anaérobiement la matière organique et forment le méthane comme sous-produit.
| Position dans la colonne | Groupe fonctionnel | Exemples d'organismes | Indicateur visuel |
| Retour au début | Photosynthétiseurs | Cyanobactéries | Couche verte ou brun-rougeâtre. Parfois des bulles d'oxygène. |
| Oxydants de soufre non photosynthétiques | Thiobacilus Beggiatoa | Couche blanche. | |
| Bactéries non sulfureuses violettes | Rhodomicrobium, Rhodospirilum, Rhodopseuodmonas | Couche rouge, violette, orange ou brune. | |
| Bactéries de soufre pourpre | Chromatium | Couche pourpre ou rouge pourpre. | |
| Bactéries vertes de soufre | Chlorobium | Couche verte. | |
| Sulfate Réduction des bactéries | Desulfovibrio, Desulfotomaculum, Desulfobacter, Desulfuromonas | Couche noire. | |
| bas | Methanogens | Méthanocoque, Methanosarcina | Parfois des bulles de méthane. |
Tableau 1 : Les principaux groupes de bactéries qui peuvent apparaître dans une colonne classique Winogradsky, de haut en bas. Des exemples d'organismes de chaque groupe sont donnés, et les indicateurs visuels de chaque couche d'organismes sont énumérés. D'après Perry et coll. (2002) et Rogan et coll. (2005).
Procédure
1. Mise en place
- Pour configurer une colonne Winogradsky, vous aurez besoin de fournitures de base :
- Une pelle, un seau et une bouteille pour recueillir les échantillons sur le terrain
- Un récipient vertical et transparent, tel qu'un cylindre gradué ou une bouteille d'eau en plastique d'environ 1 L
- Enveloppement plastique et élastiques
- grands bols à mélanger et grande cuillère à remuer
- Source de soufre (jaune d'œuf ou sulfate de calcium)
Résultats
Dans le le le cours de cette expérience, de l'eau et des sédiments ont été recueillis dans un habitat d'eau douce. Deux colonnes Winogradsky ont été construites et autorisées à se développer : une colonne Winogradsky classique incubée dans la lumière à température ambiante (Fig. 2A) et une colonne Winogradsky incubée dans l'obscurité à température ambiante (fig. 2B).

Figure 2B :
Applications et Résumé
La colonne Winogradsky est un exemple d'écosystème microbien interdépendant. Après avoir mélangé de la boue, de l'eau et des substrats de carbone et de soufre supplémentaires dans une colonne verticale, l'écosystème stratifié devrait se stabiliser en zones séparées et stables pendant plusieurs semaines. Ces zones sont occupées par différents micro-organismes qui s'épanouissent à un endroit particulier le long du gradient entre les sédiments riches en sulfures dans le fond et les sédiments riches en oxyg...
References
- Zavarzin G. (2006). Winogradsky and modern microbiology. Microbiology 75(6): 501-511. doi: 10.1134/s0026261706050018
- Esteban DJ, Hysa B, and Bartow-McKenney C (2015). Temporal and Spatial Distribution of the Microbial Community of Winogradsky Columns. PLoS ONE 10(8): e0134588. doi:10.1371/journal.pone.0134588
- Lloyd KG, Steen AD, Ladau J, Yin J, and Crosby L. (2018). Phylogenetically novel uncultured microbial cells dominate Earth microbiomes. mSystems 3(5): e00055-18. doi:10.1128/mSystems.00055-18
- Anderson DC, and Hairston RV (1999). The Winogradsky Column & Biofilms: Models for Teaching Nutrient Cycling & Succession in an Ecosystem. The American Biology Teacher, 61(6): 453-459. doi: 10.2307/4450728
- Dang H, Klotz MG, Lovell CR and Sievert SM (2019) Editorial: The Responses of Marine Microorganisms, Communities and Ecofunctions to Environmental Gradients. Frontiers in Microbiology 10(115). doi: 10.3389/fmicb.2019.00115
- Stomp M, Huisman J, Stal LJ, and Matthijs HCP. (2007) Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. ISME Journal. 1(4): 271-282. doi:10.1038/ismej.2007.59
- Perry JJ, Staley JT, and Lory S. (2002) Microbial Life, First Edition, published by Sinauer Associates
- Rogan B, Lemke M, Levandowsky M, and Gorrel T. (2005) Exploring the Sulfur Nutrient Cycle Using the Winogradsky Column. The American Biology Teacher, 67(6): 348-356. doi: 10.2307/4451860
Tags
Passer à...
Vidéos de cette collection:

Now Playing
Création d'une colonne de Winogradsky : une méthode pour enrichir les espèces microbiennes dans un échantillon de sédiments.
Microbiology
128.3K Vues

Dilutions en série et ensemencement des géloses : numération des micro-organismes
Microbiology
314.1K Vues

Cultures enrichies : Cultiver des micro-organismes aérobies et anaérobies dans des milieux sélectifs et différentiels
Microbiology
131.8K Vues

Cultures pures et ensemencement des géloses : isolement des colonies bactériennes pures à partir d'un échantillon mixte
Microbiology
165.7K Vues

Séquençage de l'ARNr 16S : une technique basée sur la PCR pour l'identification d'espèces bactériennes
Microbiology
188.0K Vues

Courbes de croissance : Générer des courbes de croissance en comptant les unités formant colonies (UFC) et en mesurant l'absorbance
Microbiology
293.3K Vues

Tests de sensibilité aux antibiotiques : Utilisation du ETEST pour déterminer la CMI de deux antibiotiques et évaluer la synergie des antibiotiques
Microbiology
93.5K Vues

Microscopie et coloration : Gram, Capsule et endospores.
Microbiology
362.5K Vues

Test de la plaque : méthode de détermination de la charge virale exprimée en unités formant des plaques
Microbiology
185.7K Vues

Transformation des cellules E. coli en utilisant le chlorure de calcium
Microbiology
86.3K Vues

Conjugaison : méthode de transfert de la résistance à l'ampicilline du donneur à l'hôte E. coli
Microbiology
38.1K Vues

Transduction via bactériophage : méthode de transfert de la résistance à l'ampicilline du donneur au receveur E. coli
Microbiology
28.9K Vues
