Soil Nutrient Analysis: Nitrogen, Phosphorus, and Potassium
Overview
Source: Laboratories of Margaret Workman and Kimberly Frye - Depaul University
In this experiment, three soil macronutrients are chemically extracted, combined with color-based reagents, then analyzed using color to determine the nutrient concentration present in the soil sample.
Nitrogen, phosphorus, and potassium are the main components of soil fertilizer. These methods isolate each nutrient from the soil into a solution that can be analyzed using turbidity and color to determine the concentration of nutrients present in the soil sample. Knowing present concentration informs environmental scientists of a nutrient deficiency or surplus in soils used to support plant production, and also provides general insight into basic biogeochemical cycles of an ecosystem.
Principles
When chemically isolated from soil, nutrients can be detected using this technique. Nitrogen and phosphorus, typically found in the form of nitrates and phosphates, are extracted with a chemical extractant that will bind the nutrient of interest. Once extracted from soil, each nutrient can be combined with a known reagent that causes the nutrient solution to change to a nutrient-specific color in a linear relationship, with a darker color indicating increased concentration of the nutrient. To analyze the concentration of each nutrient, a chemical reagent will be used to color each sample with an increase in color intensity indicating increased concentration of the nutrient.
In the high and medium-range nitrate tests, cadmium metal is used to reduce nitrates (NO3-) to nitrites (NO2-). The cadmium is contained in the purchased Nitraver 5 (high and medium range) and Nitraver 6 (low range) powder pillows.
NO3- + Cd + 2 H+ → NO2- + Cd2+ + H2O
Nitrite ions then react with sulfanilic acid (in an acidic medium contained in the NitraVer 5 powder) to form an intermediate diazonium salt. When coupled with gentisic acid (also contained in the NitraVer 5), an amber-colored solution is formed. Color intensity of this compound is directly proportional to the nitrate concentration of the water sample and can be quantified by using the nitrate color comparator box with a continuous nitrate amber color disk.
For phosphorus, sodium molydate, and potassium pyrosulfate in the purchased PhosVer 3 reagent powder react with the soluble reactive phosphates to form a phospho-molybdate complex.
H2PO4- + 12 Na2MoO4 + → PMo12O403-
The complex is then reduced by ascorbic acid (also contained in PhosVer 3 powder) to form a molybdenum blue color. The blue color is quantified using a phosphate color comparator box with a continuous phosphate blue color disk.
A color comparator box is used for this method. This tool operates based on known color intensities for each concentration between 0-50 mg/L. A color disk on the box is turned until the color in both viewing windows (blank and sample) matches. Once the colors are matched, the corresponding nutrient concentration (mg/L) will be displayed in a separate lower window on the color comparator box. These boxes are robust enough to be used with students at any level up to introductory college courses and can easily be transported as part of a field soil testing kit that can be used at a sampling location. These methods allow for basic nutrient testing in the classroom lab without requiring expensive pieces of equipment that may not be available. To ensure test accuracy, nitrate and phosphate standard solutions can be used in place of a sample in the procedures before traveling to field site or beginning analysis of soil samples in the lab.
In the potassium tests, the potassium ions combine with sodium tetraphenylborate contained in the purchased Potassium 3 reagent powder to form potassium tetraphenylborate, a white precipitate. The precipitate remains in suspension in samples, causing an increase in turbidity.
NO3- + Cd + 2 H+ NaB(C6H5)4 + K+ → KB(C6H5)4 + Na+
A potassium-measuring dipstick is used to quantify the amount of turbidity that is converted to potassium concentration. The dipstick has a black dot on one end that is placed in the sample until the dot is no longer visible through the white precipitate. The dipstick is incrementally marked to indicate a scale of visibility that is then converted to potassium concentration with a conversion chart. This method is an inexpensive procedure with minimal equipment that can be transported to an outdoor sampling site and robust enough to be used with students at any level up to introductory college courses.
Procedure
1. Extraction of Nitrogen (Nitrate NO3-)
- Turn on the balance, set a weigh boat on top, and zero the balance.
- Use a spatula to weigh out 10 g of soil (dried and sieved) and transfer to a labeled 100-mL beaker.
- Weigh out 0.1 g of calcium sulfate and transfer it to the beaker.
- Using a 25-mL graduated cylinder measure 20 mL of deionized water and transfer to the beaker.
- Repeat steps 1.1 - 1.4 for each nitrogen soil sample.
- Thoroughly mix the contents of each beaker with a stir rod.
- Secure samples on a table-top shaker and shake for 1 min.
2. Extraction of Phosphorus and Potassium
- Turn on the balance, set a weigh boat on the top, and zero the balance.
- Use a spatula to weigh out 2 g of soil (dried and sieved) and transfer into a labeled 100-mL beaker.
- Use a 25-mL graduated cylinder to measure 20 mL of Mehlich 2 soil extractant into the cylinder. Transfer to beaker.
- Repeat steps 2.1 - 2.3 for each phosphorus and potassium sample.
- Thoroughly mix the contents of each beaker with a stir rod.
- Secure samples on a table-top shaker table and shake for 5 min.
3. Nutrient Extraction Filtration - This step will be performed for all three analytes (nitrate, phosphate, and potassium)
- Secure one end of the funnel hose onto a vacuum jet.
- Secure the other end of the hose to the side arm of the flask.
- Assemble the funnel by snapping together the cylinder and perforated top disk. Place the assembled funnel on top of the side-arm flask by inserting the rubber stopper into the top of the flask to secure the funnel on top.
- Place 1 clean filter paper on top of the funnel.
- Turn on the vacuum jet.
- Slowly pour soil extract solution into the funnel, allowing the extract to drain away from the soil and into the bottom of the funnel flask.
- Pour filtered extract into a new, labeled 50-mL beaker. This filtrate will be analyzed as is.
- Remove funnel, discard filter paper, and rinse funnel and flask with deionized water. Use air jet to dry funnel and flask.
- Repeat steps 3.3 - 3.7 for each soil sample.
4. Sample Analysis with Color Comparator for Nitrate
- Label one color viewing tube “S” for sample and another color viewing tube “B” for blank.
- Thoroughly rinse both color viewing tubes with deionized water. Shake the tubes to remove the remaining rinse water.
- Add a small amount of the sample extract (prepared in steps 1.1 - 1.7) about ¼" deep to the color viewing Tube marked “S”. Cap the tube with a rubber stopper and shake it for 3 s. Discard this solution.
- Add the sample extract to both tubes until the meniscus is even with the 5-mL mark on the tubes (bottom of the frosted area).
- Add the contents of one NitraVer 5 Powder Pillow to the tube marked “S”. Cap and shake the tube vigorously for exactly one minute.
- Immediately place tubes “S” and “B” into the comparator with tube “B” in the outside hole and tube “S” in the inside hole.
- Wait 5 min, then hold the color comparator up to a light source. Rotate the disc until the color in the window for tube “B” matches the color in the window for tube “S”. Record the concentration value (mg/L) that displays in the lower window of the color comparator box.
- Repeat steps 4.1 - 4.7 for all replicates and record the average.
- Repeat step 4.8 for all nitrate samples.
5. Sample Analysis with Color Comparator for Phosphate
- Using the 2.5-mL dropper, add 2.5 mL of the filtered sample extract (prepared in steps 2.1 - 2.6) to a 25-mL graduated cylinder.
- Dilute to the 25-mL mark with deionized water, cap with stopper, and invert to mix.
- Label one color viewing tube “S” for sample and another color viewing Tube “B” for blank.
- Thoroughly rinse both color viewing tubes with deionized water. Shake the tubes to remove the remaining rinse water.
- Add a small amount of the diluted extract about ¼" deep to the color viewing tube marked “S”. Cap the tube with a rubber stopper and shake it for a few seconds then discard this solution.
- Add the sample extract to both tubes until the meniscus is even with the 5-mL mark on the tubes (bottom of the frosted area).
- Add the contents of one PhosVer 3 Powder Pillow to the “S” tube. Cap and shake the tube vigorously for one minute.
- Immediately place tubes “S” and “B” into the comparator with tube “B” in the outside hole and tube “S” in the inside hole.
- 3 min after completing Step 5.8, hold the color comparator up to a light source. Rotate the disc until the color in the window for tube “B” matches the color in the window for tube “S”. In a lower display area on the box, the color disk will simultaneously display the concentration value corresponding with the color intensity chosen. Record the concentration value that displays in the window.
- Repeat steps 5.1 - 5.10 for all replicates and record the average.
- Repeat step 5.10 for all phosphorus samples.
6. Reagent Addition and Analysis for Potassium
- Using a 1-mL dropper, add 3 mL of potassium sample extract (prepared in steps 2.1 - 2.6) to a 25-mL cylinder.
- Add DI water to the 21-mL mark on the cylinder. Firmly cap the cylinder with a rubber stopper and invert to mix.
- Add one potassium 2 reagent powder pillow to the cylinder.
- Add 3 mL of Alkaline EDTA solution to the cylinder.
- Cap the cylinder and invert several times to mix. Allow solution to stand for 3 min.
- Add the contents of one potassium 3 reagent powder pillow.
- Firmly cap the cylinder and shake vigorously for 10 s.
- Allow the solution to stand for 3 min as a white turbidity develops.
- While looking straight down into the cylinder, slowly insert the potassium dipstick vertically into the solution until black dot is no longer visible from above the cylinder.
- Hold dipstick in that position and rotate the cylinder so it can be seen the scale on the dipstick. Look across the surface of the scale on the dipstick. Record the number on the dipstick scale where the surface of the sample meets the dipstick scale.
- Repeat 6.1- 6.10 for all replicates and average. Repeat 6.11 for all potassium samples.
- Refer to the potassium conversion table to determine the concentration of potassium in soil samples. Locate the dipstick reading on the left column and record the corresponding mg/L concentration on the right column.
Results
Each nutrient analysis will result in a concentration reported in mg/L.
Nitrate and Phosphate concentrations will be determined with the color comparator boxes and display the result in the window.

Figure 1. Example color disks for nitrate (left) and phosphate (right) color comparator boxes. Color intensities are on the outer edge of the disks and nutrient concentration (mg/L) are on the inner edge of the disks.
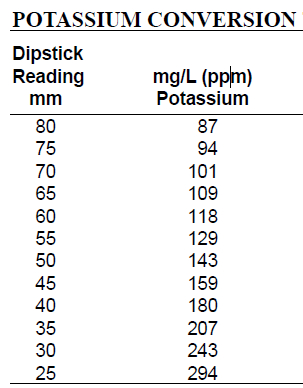
Table 1. Potassium Conversion Table used to convert dipstick potassium reading into mg/L. Locate the dipstick reading on the left column and record the corresponding mg/L concentration on the right column.
| Nitrogen | Phosphorus | Potassium | |
| Nutrient level range in ppm | |||
| Low | 0-15 | 0-25 | 0-60 |
| Medium | 15-30 | 25-50 | 60-100 |
| High | 30+ | 50+ | 100+ |
Table 2. Table of nutrient ranges arranged by categories.
Application and Summary
Determining the nutrient concentrations for nitrate, phosphates, and potassium can reveal how a soil is functioning in regards to its intended use and how nutrients are cycling through a soil. A nutrient test provides a report of average nutrient concentration (mg/L) for all nutrients tested. In an agricultural setting, knowing the concentration of nutrients can help food producers know when to add fertilizer, how much to add, and which nutrients need supplemented and in what amount. Consistently high nitrogen soils, for instance, would be good for growing nitrogen-demanding crops such as soy and corn. High nitrogen levels are also particularly useful for non-flowering plants because nitrogen is required for any green part of plants. High nitrogen levels can suppress flowering however, if they remain higher than phosphorus levels. Phosphorus controls flowering in plants and is important to any plant production involving flowering or fruiting plants and phosphorus is often added to soils or directly to plants before and during flowering and fruiting life-cycle stages to increase agricultural yields in larger crop size and increased amounts of fruit production per plant. Potassium is involved in catalyzing many chemical reactions required to support plant life including drought tolerance and moisture regulation. Low potassium soils will likely need to be irrigated if soil amendment is not possible. Nutrient concentration can also inform of nutrient deficiencies or surpluses that can be detrimental to plant growth. If a nutrient is too high, amendments can be performed to reduce a surplus, such as adding mulch or tilling the soil. If nutrients are too low to support plant production, fertilization can be used to add nutrients in an amount needed for a specific crop. Low nutrient soil may also have more applicable uses to land managers for recreational or developed (paved surfaces or building construction) spaces.
Tags
Skip to...
Videos from this collection:

Now Playing
Soil Nutrient Analysis: Nitrogen, Phosphorus, and Potassium
Environmental Science
216.3K Views

Tree Identification: How To Use a Dichotomous Key
Environmental Science
81.4K Views

Tree Survey: Point-Centered Quarter Sampling Method
Environmental Science
49.6K Views

Using GIS to Investigate Urban Forestry
Environmental Science
12.8K Views

Proton Exchange Membrane Fuel Cells
Environmental Science
22.2K Views

Biofuels: Producing Ethanol from Cellulosic Material
Environmental Science
53.8K Views

Testing For Genetically Modified Foods
Environmental Science
90.1K Views

Turbidity and Total Solids in Surface Water
Environmental Science
35.9K Views

Dissolved Oxygen in Surface Water
Environmental Science
56.0K Views

Nutrients in Aquatic Ecosystems
Environmental Science
39.2K Views

Measuring Tropospheric Ozone
Environmental Science
26.5K Views

Determination Of NOx in Automobile Exhaust Using UV-VIS Spectroscopy
Environmental Science
30.4K Views

Lead Analysis of Soil Using Atomic Absorption Spectroscopy
Environmental Science
126.2K Views

Carbon and Nitrogen Analysis of Environmental Samples
Environmental Science
29.6K Views

Analysis of Earthworm Populations in Soil
Environmental Science
16.6K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved