Ozonolysis of Alkenes
Overview
Source: Vy M. Dong and Zhiwei Chen, Department of Chemistry, University of California, Irvine, CA
This experiment will demonstrate an example of an ozonolysis reaction to synthesize vanillin from isoeugenol (Figure 1). Ozonolysis of alkenes, an oxidation reaction between ozone and an alkene, is a common method to prepare aldehydes, ketones, and carboxylic acids. This experiment also demonstrates the use of an ozone generator and a low temperature (−78 °C) reaction.
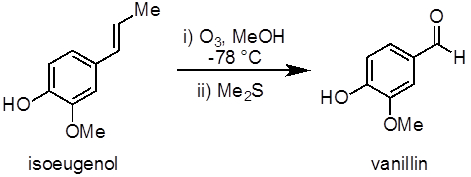
Figure 1. Diagram showing the ozonolysis of isoeugenol to vanillin.
Procedure
- Add 200 mg of isoeugenol, 15 mL of MeOH, and ~ 2 mg of Sudan III to a 3-necked 50- mL round bottom flask equipped with a magnetic stir bar.
- Connect the reaction flask to an oxygen tank and a bubbler.
- Turn on the flow of oxygen.
- Cool the reaction mixture with a dry ice/acetone bath.
- Switch on the ozone generator, which converts the oxygen from the tank to ozone that goes into the reaction flask. The generator will be between the oxygen tank and the reaction flask. Allow the reaction mixture to st
Results
Application and Summary
In this experiment, we have demonstrated the synthesis of vanillin from isoeugenol using the ozonolysis reaction. Also, using an ozone generator while performing a low temperature reaction was shown.
Ozonolysis is a useful reaction to prepare aldehydes, ketones, and carboxylic acids from alkenes. It has been applied in natural product synthesis and industrial-scale preparation of pharmaceuticals. Artemisinin is a potent antimalarial agent and was one of the nat
Tags
Skip to...
Videos from this collection:

Now Playing
Ozonolysis of Alkenes
Organic Chemistry II
66.8K Views

Cleaning Glassware
Organic Chemistry II
123.2K Views

Nucleophilic Substitution
Organic Chemistry II
99.2K Views

Reducing Agents
Organic Chemistry II
42.9K Views

Grignard Reaction
Organic Chemistry II
148.8K Views

n-Butyllithium Titration
Organic Chemistry II
47.7K Views

Dean-Stark Trap
Organic Chemistry II
99.9K Views

Organocatalysis
Organic Chemistry II
16.6K Views

Palladium-Catalyzed Cross Coupling
Organic Chemistry II
34.2K Views

Solid Phase Synthesis
Organic Chemistry II
40.9K Views

Hydrogenation
Organic Chemistry II
49.5K Views

Polymerization
Organic Chemistry II
93.7K Views

Melting Point
Organic Chemistry II
149.6K Views

Infrared Spectroscopy
Organic Chemistry II
214.2K Views

Polarimeter
Organic Chemistry II
99.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved