Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique
Overview
Source: Tamara M. Powers, Department of Chemistry, Texas A&M University
Inorganic chemists often work with highly air- and water-sensitive compounds. The two most common and practical methods for air-free synthesis utilize either Schlenk lines or gloveboxes. This experiment will demonstrate how to perform simple manipulations on a Schlenk line with a focus on solvent preparation and transfer. Through the synthesis of a reactive Ti(III) metallocene complex, we will demonstrate a new, simple method to degas solvent as well as how to transfer solvent by cannula and by syringe on a Schlenk line.
The synthesis of a Ti(III) metallocene compound 3 is shown in Figure 1.1 Compound 3 is highly reactive with O2, (see oxidation of compound 3 to Ti(IV) metallocene 4 shown in Figure 1). Therefore, it is important to run the synthesis under anaerobic conditions. The synthesis of target compound 3 can be monitored visually and progresses through one additional color change before arriving at the desired product, which is blue in color. If during the experiment there is an observed color change from blue to yellow (or green = blue + yellow), this is an indication that O2 entered the flask and that undesired oxidation of compound 3 to the Ti(IV) analog (compound 4) has occurred.
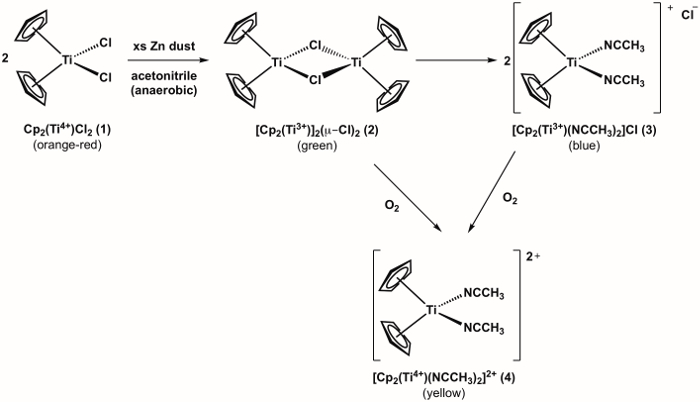
Figure 1. Synthesis of Ti(III) metallocene compound 3 and it's reaction with O2.
Procedure
1. Setup of the Schlenk Line
For a more detailed procedure, please review the "Schlenk Lines Transfer of Solvent" video in the Essentials of Organic Chemistry series. Schlenk line safety should be reviewed prior to conducting this experiment. Glassware should be inspected for star cracks before use. Care should be taken to ensure that O2 is not condensed in the Schlenk line trap if using liquid N2. At liquid N2 temperature, O2 condense
Results
Upon addition of the acetonitrile in step 4, the solution should change color from orange, to green, to blue (Figure 4). Failure to obtain the blue color indicates a leak in the system. Addition of acetonitrile by syringe in step 6 should result in no color change if anaerobic conditions are maintained. If oxygen is present, the solution will turn from blue, to green, to orange.
Application and Summary
Here, we demonstrated standard Schlenk line technique to synthesize an air-sensitive Ti(III) metallocene complex. The solvent was degassed by bubbling N2 through the liquid in a Schlenk flask. We also demonstrated how to set up a reaction under anaerobic conditions on the Schlenk line and transfer solvent anaerobically by cannula transfer as well as by syringe.
Inorganic chemists use Schlenk line technique in the synthesis of air- and water-sensitive compounds. The solvent used in t
References
- Burgmayer, S. N. Use of a Titanium Metallocene as a Colorimetric Indicator for Learning Inert Atmosphere Techniques. J Chem Educ. 75, 460 (1998).
Skip to...
Videos from this collection:

Now Playing
Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique
Inorganic Chemistry
31.5K Views

Glovebox and Impurity Sensors
Inorganic Chemistry
18.6K Views

Purification of Ferrocene by Sublimation
Inorganic Chemistry
54.4K Views

The Evans Method
Inorganic Chemistry
68.3K Views

Single Crystal and Powder X-ray Diffraction
Inorganic Chemistry
104.2K Views

Electron Paramagnetic Resonance (EPR) Spectroscopy
Inorganic Chemistry
25.4K Views

Mössbauer Spectroscopy
Inorganic Chemistry
22.0K Views

Lewis Acid-Base Interaction in Ph3P-BH3
Inorganic Chemistry
38.8K Views

Structure Of Ferrocene
Inorganic Chemistry
79.3K Views

Application of Group Theory to IR Spectroscopy
Inorganic Chemistry
45.1K Views

Molecular Orbital (MO) Theory
Inorganic Chemistry
35.2K Views

Quadruply Metal-Metal Bonded Paddlewheels
Inorganic Chemistry
15.3K Views

Dye-sensitized Solar Cells
Inorganic Chemistry
15.7K Views

Synthesis of an Oxygen-Carrying Cobalt(II) Complex
Inorganic Chemistry
51.6K Views

Photochemical Initiation Of Radical Polymerization Reactions
Inorganic Chemistry
16.7K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved