3.13 : Introduction to Enzyme Kinetics
Enzyme kinetics studies the rates of biochemical reactions. Scientists monitor the reaction rates for a particular enzymatic reaction at various substrate concentrations. Additional trials with inhibitors or other molecules that affect the reaction rate may also be performed.
The experimenter can then plot the initial reaction rate or velocity (Vo) of a given trial against the substrate concentration ([S]) to obtain a graph of the reaction properties. For many enzymatic reactions involving a single substrate, this data fits the Michaelis-Menten equation, an equation derived by Leonor Michaelis and Maud Menten.
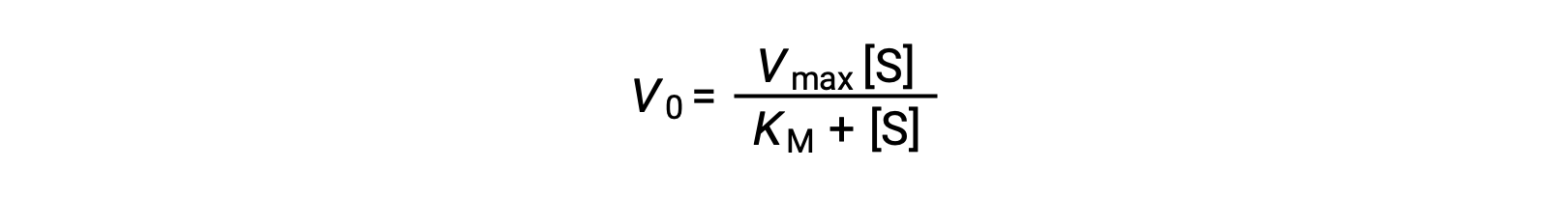
The equation estimates the maximum velocity (Vmax) and the Michaelis constant (KM) for the enzyme being studied and is based on the following assumptions:
- No product is present at the start of the reaction.
- The rate of enzyme-substrate complex formation equals the rate of dissociation and breakdown into products.
- The enzyme concentration is minimal compared to the substrate concentration.
- Only the initial reaction rates are measured.
- The enzyme is present either in the free form or in the enzyme-substrate complex.
Different rearrangements of the Michaelis-Menten equation, such as the Lineweaver-Burke, Eadie-Hofsteot, and Hanes-Woolf plots, are alternate ways to graph kinetic parameters. The Lineweaver-Burke or double reciprocal plot is often used to estimate the KM and the Vmax. The plot uses the reciprocals values of the x and y-axis from the Michaelis-Menten plot. Mathematically, the y-intercept equals 1/Vmax, and the x-intercept equals −1/KM.
The Lineweaver-Burke plot can be used to visually differentiate between inhibitor types – competitive, non-competitive, and uncompetitive. Different rearrangements of the Michaelis-Menten equation, such as the Eadie-Hofstee and Hanes-Woolf plots, are also used to determine kinetic parameters.
From Chapter 3:

Now Playing
3.13 : Introduction to Enzyme Kinetics
Energy and Catalysis
17.5K Views

3.1 : The First Law of Thermodynamics
Energy and Catalysis
4.7K Views

3.2 : The Second Law of Thermodynamics
Energy and Catalysis
4.3K Views

3.3 : Enthalpy within the Cell
Energy and Catalysis
4.7K Views

3.4 : Entropy within the Cell
Energy and Catalysis
8.2K Views

3.5 : An Introduction to Free Energy
Energy and Catalysis
6.6K Views

3.6 : Endergonic and Exergonic Reactions in the Cell
Energy and Catalysis
12.6K Views

3.7 : The Equilibrium Binding Constant and Binding Strength
Energy and Catalysis
8.2K Views

3.8 : Free Energy and Equilibrium
Energy and Catalysis
5.0K Views

3.9 : Non-equilibrium in the Cell
Energy and Catalysis
3.1K Views

3.10 : Oxidation and Reduction of Organic Molecules
Energy and Catalysis
5.1K Views

3.11 : Introduction to Enzymes
Energy and Catalysis
14.7K Views

3.12 : Enzymes and Activation Energy
Energy and Catalysis
10.2K Views

3.14 : Turnover Number and Catalytic Efficiency
Energy and Catalysis
9.0K Views

3.15 : Catalytically Perfect Enzymes
Energy and Catalysis
3.6K Views
See More
ABOUT JoVE
Copyright © 2025 MyJoVE Corporation. All rights reserved