11.6 : Column Efficiency: Plate Theory
Band broadening in a chromatography column is measured by its efficiency. This is determined by the number of theoretical plates (N). Theoretical plate theory states that a separation column consists of a continuous series of imaginary plates where solute equilibration occurs between stationary and mobile phases.
A higher number of theoretical plates signifies better column efficiency and improved separation capabilities. Plate height affects bandwidth and separation quality; it is inversely proportional to column efficiency. The number of theoretical plates (N) is calculated as the column length (L) divided by plate height (H), as shown in the equation:
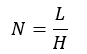
Minimizing H is crucial for achieving better efficiency and reducing column length.
Plate heights vary for different solutes due to differing diffusion coefficients. They range from 0.1 to 1 mm in gas chromatography, around 10 µm in high-performance liquid chromatography, and less than 1 µm in capillary electrophoresis.
Column efficiency can be defined as the variance per unit length, as chromatographic bands often have Gaussian shapes. The number of theoretical plates also relates to retention time and peak width at the base or half height. It is important to note that theoretical plates are an abstract concept, and their number depends on the column and solute properties, making them variable for different solutes.
In packed columns, the height equivalent to a theoretical plate (HETP) is a valuable parameter representing column performance. HETP indicates the column length needed for one theoretical plate and is essential for designing and evaluating packed column efficiency. A lower HETP value signifies a higher column efficiency and improved separation capabilities.
From Chapter 11:

Now Playing
11.6 : Column Efficiency: Plate Theory
Principles of Chromatography
446 Views

11.1 : Chromatographic Methods: Terminology
Principles of Chromatography
906 Views

11.2 : Chromatographic Methods: Classification
Principles of Chromatography
1.1K Views

11.3 : Analyte Adsorption and Distribution
Principles of Chromatography
552 Views

11.4 : Diffusion on Chromatography Columns
Principles of Chromatography
402 Views

11.5 : Chromatographic Resolution
Principles of Chromatography
329 Views

11.7 : Column Efficiency: Rate Theory
Principles of Chromatography
232 Views

11.8 : Optimizing Chromatographic Separations
Principles of Chromatography
306 Views

11.9 : Silica Gel Column Chromatography: Overview
Principles of Chromatography
938 Views

11.10 : Thin-Layer Chromatography (TLC): Overview
Principles of Chromatography
1.0K Views

11.11 : Gas Chromatography: Introduction
Principles of Chromatography
816 Views

11.12 : Gas Chromatography: Types of Columns and Stationary Phases
Principles of Chromatography
412 Views

11.13 : Gas Chromatography: Sample Injection Systems
Principles of Chromatography
325 Views

11.14 : Gas Chromatography: Overview of Detectors
Principles of Chromatography
342 Views

11.15 : Gas Chromatography: Types of Detectors-I
Principles of Chromatography
324 Views
See More
Copyright © 2025 MyJoVE Corporation. All rights reserved