Introduction to Titration
Overview
Source: Laboratory of Dr. Yee Nee Tan — Agency for Science, Technology, and Research
Titration is a common technique used to quantitatively determine the unknown concentration of an identified analyte.1-4 It is also called volumetric analysis, as the measurement of volumes is critical in titration. There are many types of titrations based on the types of reactions they exploit. The most common types are acid-base titrations and redox titrations.5-11
In a typical titration process, a standard solution of titrant in a burette is gradually applied to react with an analyte with an unknown concentration in an Erlenmeyer flask. For acid-base titration, a pH indicator is usually added in the analyte solution to indicate the endpoint of titration.12 Instead of adding pH indicators, pH can also be monitored using a pH meter during a titration process and the endpoint is determined graphically from a pH titration curve. The volume of titrant recorded at the endpoint can be used to calculate the concentration of the analyte based on the reaction stoichiometry.
For the acid-base titration presented in this video, the titrant is a standardized sodium hydroxide solution and the analyte is domestic vinegar. Vinegar is an acidic liquid that is frequently used as a culinary condiment or flavorings. Vinegar mainly consists of acetic acid (CH3COOH) and water. The acetic acid content of commercial vinegar can vary widely and the goal of this experiment is to determine the acetic acid content of commercial vinegar by titration.
Principles
The determination of acetic acid in vinegar is based on the principle of an acid-base titration method. The reaction between NaOH and CH3COOH is shown in Equation 1:
CH3COOH(aq) + NaOH(aq) → H2O(l) + NaCH3CO2(aq) (1)
The standardized NaOH solution is progressively added to the vinegar with unknown acetic acid concentration until the end point is reached. During the acid-base titration, the pH can be plotted as a function of the volume of the titrant added. The inflection point on the curve, the point at which there is a stoichiometric equal amount of acid and base in a solution, is called the equivalence point. Most acids and bases are colorless, with no visible reaction occurring at the equivalence point. To observe when the equivalence point has been reached, a pH indicator is added. The endpoint is not the equivalence point but a point at which the pH indicator changes color. It is important to select an appropriate pH indicator so that the end point is as close to the equivalence point of titration as possible.
At the end point of this reaction, the conjugate base NaCH3CO2 is slightly basic. Phenolphthalein indicator has a working pH range of 8.3–10.0, which is colorless in acidic solution and magenta above pH 8.2. Therefore, phenolphthalein is a preferred indicator as it will change from colorless to pink at this condition. When performing the experiment, it is best to keep the concentration of pH indicator low because pH indicators themselves are usually weak acids that react with base.
The volume of standardized NaOH solution added at end point can then be used to calculate the molar concentrations of acetic acid based on the stoichiometry of the above equation. In this experiment, the titrant NaOH is a strong alkaline and the analyte acetic acid is a weak acid.
Before performing the experiment, it is important to consider the hygroscopic nature of NaOH. This property requires its solution to be standardized with a stable primary standard such as potassium hydrogen phthalate (KHC8H4O4). The exact molar concentration of NaOH solution can then be accurately determined after standardization. The reaction between the primary acid standard and NaOH is shown in Equation 2:
KHC8H4O4(aq) + NaOH(aq) → H2O(l) + NaKC8H4O4(aq) (2)
A detailed step-by-step titration protocol is presented in the following section.
Procedure
1. Standardization of NaOH with Potassium Hydrogenphthalate (KHC8H4O4)
- To begin, the titrant, sodium hydroxide, must be standardized. Prepare a stock NaOH solution by dissolving about 4 g of NaOH pellet in 100 mL of deionized water. Note that NaOH is a hazardous chemical which is corrosive to skin and irritant to eye, be cautious and wear proper personal protection equipment (PPE) to avoid skin or eye contact.
- Make a 1:10 dilution of the sodium hydroxide solution by adding 25 mL of the stock sodium hydroxide solution to a 500-mL bottle. Sodium hydroxide absorbs carbon dioxide. It is important to prevent this by making sure to use boiled, deionized water, an oven-dried bottle, and to cap the bottle quickly. Make the solution up to 250 mL with the deionized water and shake to mix.
- Dry 4–5 g of the primary standard acid, KHC8H4O4 at 110 °C for 4 h in a drying oven and then cool the solid in a desiccator for 1 h.
- Dissolve about 4 g of dried KHC8H4O4 in 250 mL of deionized water. Record the mass accurately. Calculate the molar concentration of the KHC8H4O4 solution.
- Pipette 25 mL of KHC8H4O4 into a clean and dry Erlenmeyer flask. Add 2 drops of phenolphthalein, and swirl gently to mix well. Note that phenolphthalein is toxic and irritant, be cautious to avoid skin or eye contact.
- Clean a 50-mL burette and a funnel thoroughly with detergent and water. Flush the burette with water and rinse 3x with deionized water. Rinse the burette with the diluted NaOH solution 3x, making sure that the NaOH wets the entire inner surface and drain the waste through the tip. Mount the washed burette on a ringstand with a clamp and ensure that it stands vertically.
- Fill the clean burette with the diluted NaOH solution. It should be noted that the amount of the diluted NaOH needs not be exactly at the zero mark but should be within the scale and sufficient for at least one titration. Air bubbles may affect the accuracy of volume reading. Carefully check the burette for air bubbles, and gently tap the burette to free them and open the stopcock to let a few mL of titrant to flow through and at the same time releasing any trapped air. Read the volume by viewing the bottom of the meniscus after 10 s. Record this initial volume. Pay attention to the significant figures of the reading. Record the value to two decimal places in mL.
- Place the Erlenmeyer flask containing potassium hydrogen phthalate (KHC8H4O4) under the burette and adjust the height of the burette properly. Titrate the KHC8H4O4 solution by slowly adding NaOH solution in 1–2 mL increments using one hand to control the flow rate by adjusting the stopcock, and the other swirling the flask.
- When close to the endpoint, begin adding the titrant drop by drop. The endpoint is reached when the solution turns a faint, persistent pink color. Record the final volume of the diluted NaOH in the burette.
- Repeat the titration at least twice more to obtain consistent data. Calculate the molar concentration of the diluted NaOH solution.
2. Titration of Vinegar with Standardized Sodium Hydroxide Solution
- The sodium hydroxide solution is now standardized and can be used as a titrant to analyze vinegar. To reduce the pungent aroma of vinegar, dilute 10 mL of the vinegar solution to be tested in a 1:10 ration to a total volume of 100 mL.
- Pipette 25 mL of analyte, to a clean and dry Erlenmeyer flask (noted as VA). Add 2 drops of phenolphthalein.
- Fill the burette with the standardized NaOH solution from the first part of the Procedure. Record the initial volume of titrant (V1).
- Progressively add the standardized NaOH solution to the vinegar. When the volume of titrant approaches the expected value, adjust the stopcock to add the titrant drop by drop. Continue to swirl the flask with one hand and keep the other hand ready to close the stopcock. Once the analyte solution changes to light pink color, swirl for a few seconds to see whether the color will fade. If the color persists, the titration reaches the end point. Record the final volume of titrant (V1'). If the solution color fades, add one more drop of titrant. Wash the bottom tip of the burette using the wash bottle. Collect the washed mixture and watch the color change of the analyte solution. Continue the titration till the end point. Record the amount of titrant needed (Vt1 = V1' V1).
- Repeat the titration at least twice until three concordant values that are within 0.1 mL of one another is obtained (Vt2 and Vt3).
- Calculate the mean value of titrant volume using the three values obtained in three different titrations: Vt = (Vt1 + Vt2 + Vt3)/3. The molar concentration of acetic acid in vinegar can be thus calculated using Equation 3.
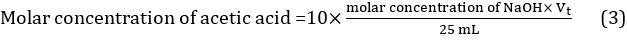
Results
| Unit | Trial 1 | Trial 2 | Trial 3 | |||
| Volume of diluted vinegar acid (VA) | mL | 25.00 | ||||
| Molar concentration of NaOH (cNaOH) | mol/L | 0.09928 | ||||
| Initial burette reading of NaOH | mL | 0.10 | 0. 05 | 1.20 | ||
| Final burette reading of NaOH | mL | 18.75 | 18.60 | 19.80 | ||
| Volume of NaOH dispensed | mL | 18.65 | 18.55 | 18.60 | ||
| Mean volume of NaOH dispensed (Vt) | mL | 18.60 | ||||
Table 1. Titration results.
Sample calculations:
Mass of KC8H5O4 = 4.0754 g
Molar mass of KC8H5O4 = 204.22 g/mol
Number of moles of KC8H5O4 in 25.00 mL standard solution = 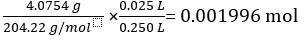
According to Equation 2,
Concentration of the diluted NaOH solution = 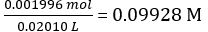
Moles of NaOH dispensed = concentration of NaOH × mean volume of NaOH dispensed = 0.09928 mol/L × 18.60 mL = 1.847 × 10-3 mol
According to Equation 1,
Number of moles of CH3COOH in 25.00 mL of diluted vinegar = 1.847 × 10-3 mol
Concentration of diluted vinegar = 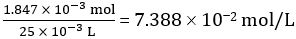
Hence concentration of undiluted vinegar = 10 × 7.388 102 mol/L = 0.7388 mol/L
The above steps are presented to illustrate the calculation procedure; we can simply apply Equation 3 to obtain the concentration of undiluted vinegar in one step.
Therefore 1.000 L of undiluted vinegar contains 0.7388 mol of CH3COOH.
Volume of CH3COOH=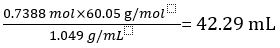
Volume percent of vinegar = 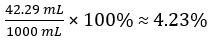
Application and Summary
Titration is an important chemical method that is frequently applied in current chemistry research. For example, acid base titration is applied to determine amine or hydroxyl value of a sample. The amine value is defined as the number of milligrams of KOH equivalent to the amine content in one gram of sample. To determine the hydroxyl value, the analyte is first acetylated using acetic anhydride then titrated with KOH. The mass in milligrams of KOH then corresponds to hydroxyl groups in one gram of sample.13 Another example is the Winkler test, a specific type of redox titration used to determine the concentration of dissolved oxygen in water for water quality studies. Dissolved oxygen is reduced using manganese(II) sulfate, which then reacts with potassium iodide to produce iodine. Since the iodine released is directly proportional to the oxygen content, the oxygen concentration is determined by titrating iodine with thiosulfate using a starch indicator.14
Besides applications in basic chemical research, titration has also been widely adopted in industrial and everyday use. In biodiesel industry, waste vegetable oil (WVO) must first be neutralized to remove free fatty acids that would normally react to make undesired soap. A portion of WVO is titrated with a base to determine the sample acidity, so the rest of the batch could be properly neutralized.15 Benedict's method, a test for quantification of urine glucose level, is another example showing the importance of titration in healthcare. In this titration, cupric ions are reduced to cuprous ions by glucose, which then react with potassium thiocyanate to form a white precipitate, indicating the endpoint.16
Tags
Skip to...
Videos from this collection:

Now Playing
Introduction to Titration
General Chemistry
425.8K Views

Common Lab Glassware and Uses
General Chemistry
659.5K Views

Solutions and Concentrations
General Chemistry
275.6K Views

Determining the Density of a Solid and Liquid
General Chemistry
557.1K Views

Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
383.9K Views

Determining the Empirical Formula
General Chemistry
183.9K Views

Determining the Solubility Rules of Ionic Compounds
General Chemistry
141.6K Views

Using a pH Meter
General Chemistry
347.0K Views

Ideal Gas Law
General Chemistry
79.5K Views

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
158.9K Views

Le Châtelier's Principle
General Chemistry
265.9K Views

Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
160.9K Views

Determining Rate Laws and the Order of Reaction
General Chemistry
196.5K Views

Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.8K Views

Coordination Chemistry Complexes
General Chemistry
91.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved