Method Article
Using Reference Reagents to Confirm Robustness of Cytokine Release Assays for the Prediction of Monoclonal Antibody Safety
In This Article
Summary
The use of cytokine release assay reference reagents allows for more reproducible and standardized in vitro safety profiles of immunotherapeutic monoclonal antibodies. Here we describe how cytokine release assays can be used alongside a reference reagent panel to predict the safety of some therapeutic monoclonal antibodies.
Abstract
New immunostimulatory antibody drugs designed to either directly stimulate specific immune cells or indirectly enhance the immune response by blocking or activating an endogenous regulator of the immune system have the potential to cause serious immune-related adverse events such as cytokine release syndrome (CRS). It is, therefore crucial to assess the safety profile of such drugs with a combination of in vivo and in vitro experiments before first-in-human dose administration. Cytokine release assays (CRAs), where the proposed antibody therapeutic is co-cultured with human immune cells (such as peripheral blood mononuclear cells (PBMCs), whole blood, or otherwise) and the amount of inflammatory cytokine produced is measured, are critical for hazard identification. However, different labs using different control antibodies can threaten the harmonization of CRAs, and clinically relevant controls (such as TGN1412) can be difficult to source, which can lead to less accurate or reliable results or data which are difficult to compare between laboratories. The inclusion of positive and negative controls in a CRA can ensure the accuracy and reliability of the results. The National Institute for Biological Standards and Control (NIBSC) has produced a panel of lyophilized antibody controls intended for use in various CRA platforms to harmonize results across various laboratories and assay methods. A set of three different positive control antibodies include anti-CD52, anti-CD3, and anti-CD28 superagonist (SA), which are known to induce dose-dependent CRS in patients. Each antibody is provided with an isotype-matched negative control antibody. This panel of reference reagents has previously been shown to have good inter-lab reproducibility and are suitable controls to increase the confidence and robustness of safety data from a variety of CRA platforms.
Introduction
Induced cytokine release can be an anticipated and desired effect of some immunomodulatory monoclonal antibodies (mAbs). However, the unanticipated release of pro-inflammatory cytokines can result in cytokine release syndrome (CRS) in patients characterized by fever, fatigue, and even multiple organ failure1. It is, therefore crucial that new immunostimulatory mAbs are tested in vitro for their potential to cause CRS by measuring the release of cytokines in a cytokine release assay (CRA).
TGN1412 is a monoclonal antibody that was developed as a potential treatment for B cell chronic lymphocytic leukemia by acting as a CD28 superagonist (CD28SA), capable of activating T-lymphocytes by crosslinking the co-stimulatory receptor CD282. In 2006, six healthy volunteers who were administered TGN1412 in a clinical trial experienced severe side effect, including CRS, within hours of receiving the drug1. This led to the trial being stopped and TGN1412 being withdrawn from further development. Other antibodies which are known to cause CRS as a side effect include the anti-CD52 mAb, Campath-1H3, and the anti-CD3 mAb, Muromonab (OKT3)4. Given their ability to induce dose-dependent CRS in patients, TGN1412, OKT3, and Campath-1H are suitable positive control antibodies for the generation of robust and reliable results from a CRA. Yet, these control antibodies were not previously easily obtainable because of high costs or restricted availability.
However, a remanufactured lyophilized reference panel of these three antibodies5,6,7 alongside isotype-matched negative controls was recently made available by the National Institute for Biological Standards and Control (NIBSC). This panel of reference reagents has previously been shown to have good inter-laboratory reproducibility8 and is, therefore a suitable control to increase the confidence and robustness of safety data from a variety of CRA platforms. Thus, the rationale behind the use of this protocol alongside these reagents is to improve upon CRA harmonization, with the advantage of these reagents having been validated in an international collaborative study8.
Here we describe how best to use this panel of reference reagents in a solid-phase (SP) PBMC CRA and an aqueous-phase (AQ) whole blood (WB) CRA to predict antibody-induced CRS. Both of these CRA formats are complementary due to the mode of antibody presentation (indirect for the solid phase versus direct for the aqueous phase) and due to the fact that they target different groups of responder cells. The same protocol may be adapted for use with diluted whole blood (dWB) or with endothelial: PBMC co-cultures.
Protocol
The following protocol follows the guidelines of NIBSC research ethics committee. In accordance with applicable regulations and guidelines, obtain written informed consent of healthy donor or patients. Use sterile techniques to perform all the preparation steps of the protocol in a laminar flow cell culture hood. See Table of Materials for details about all reagents and equipment.
1. Preparation of coated plates with mAb for solid phase (SP) assay
- Reconstitute contents of the reference reagent ampoules with 1 mL of sterile distilled water. Allow 5-10 min for rehydration before mixing the antibody solution and transferring to a sterile capped tube.
- After reconstitution in 1 mL of water the stock concentration of the following recombinant antibodies will be 200 µg/mL: anti-CD3 (15/162), anti-CD52 (15/178), IgG1K isotype control (15/198) for anti-CD52, IgG2a isotype control (15/218) for anti CD3, IgG4 isotype control (15/232) for anti-CD28SA.
- After reconstitution in 1 mL of water the stock concentration of anti-CD28SA (15/144) will be 100 µg/mL. For short term storage up to 7 days, transfer the reconstituted material in a sterile capped tube to 4 °C.
- Dilute the reconstituted antibodies and test antibodies to 10 µg/mL in sterile PBS and coat the wells of a sterile non-TC treated U-bottom polypropylene 96-well microtiter plate with 100 µL of diluted antibody solution (1 µg/well) and incubate overnight at 4 °C.
NOTE: It is important to use polypropylene plates for protein adsorption, for these were used in the validation of the standard reagents9.
2. Preparation of PBMCs
- Collect a minimum of 30 mL of peripheral whole blood (WB) into heparinized/heparin containing tubes and invert several times to ensure proper mixture with the sodium heparin.
- Transfer 15 mL of WB in a separate tube to be used later in the preparation of aqueous phase whole blood assay (step 3.1)
- Dilute the remaining 15 mL of blood in a 1:1 volume ratio with PBS or serum-free RPMI-1640 media and gently layer the diluted blood over the top of 15 mL of density gradient medium (e.g., lymphoprep, Ficoll-Hypaque) in a 50 mL tube.
- Centrifuge the tube at 500 x g for 20 min at room temperature in a swing-out rotor with no brake and with a reduced acceleration to separate the blood into its different components.
- After centrifugation, the density gradient will separate as a top layer of plasma followed by a thin layer of buffy coat containing PBMCs and a bottom layer containing red blood cells (RBCs) and polymorphonuclear granulocytes including neutrophils and eosinophils. Carefully harvest the PBMCs by inserting a pipette directly through the upper plasma layer to the PBMCs. Alternatively, remove the upper layer prior to collection of cells.
- Gently resuspend the buffy coat in 10 mL of PBS or serum-free RPMI-1640 media. Centrifuge the tube again at 500 x g for 10 min to pellet the cells. Remove the supernatant and discard it.
- Repeat wash step 2.6. and resuspend pellet in 2 mL of RPMI with 10% FCS (complete RPMI-1640, cRPMI)
- Count cells using a haemocytometer10.
- Adjust PBMCs to a concentration of 1 x 106 cells/mL in cRPMI.
3. Preparation of aqueous phase (AQ) whole blood (WB) cytokine release assay
- Add 190 µL of WB to the wells of a 96-well U-bottom polystyrene plate. If not already at 100 µg/mL, pre-dilute all treatment antibodies and reference reagents to 100 µg/mL in PBS.
- Add 10 µL of diluted antibodies to 190 µL of WB to give a final antibody concentration of 5 µg/mL antibody in 95% WB.
- Incubate plate for 48 h in a humidified incubator at 37 °C.
4. Preparation of solid phase (SP) PBMC cytokine release assay
- With a multichannel pipette remove and discard the antibody solution from the coated plates (described in step 1), fill a reagent reservoir with PBS and wash the plate 3x with 200 µL of PBS to remove unbound mAbs.
- Add 200 µL of the cell suspension from step 2.8 to each well. Incubate the plate for 48 h in a humidified incubator at 37 °C, 5% CO2.
5. Collection of supernatant or plasma
- After incubation for 48 h with control and test mAbs, centrifuge plates at 400 x g for 5 min and collect the cell-conditioned medium or plasma, taking care to not disturb the cell pellet. Freeze the collected supernatant or plasma at -20 °C.
NOTE: Make sure not to disturb the red blood cell pellet when collecting the plasma.
6. Performing quantification of cytokines in the supernatant or plasma
- Using the collected supernatant or plasma, perform cytokine analysis of IFN-γ, IL-2, TNF-α and IL-6 concentrations using the preferred multiplex option. An example method of multiplex cytokine analysis using a cytometric bead assay has previously been published11.
NOTE: Please refer to the Table of Materials for the multiplex kit which was used in the generation of the representative results.
Results
The results of the SP assay should demonstrate a release of IL-2, IFN-γ, IL-6 and TNF-α12 in the pg/mL range from all positive control antibodies at 48 h and should be statistically significantly greater than matched isotype controls. Our representative results demonstrate that the positive control antibodies, αCD28, αCD3 and αCD28SA induce significantly high levels of IFN-γ , IL-6 and TNF-α in comparison to matched isotype controls, when run in the PBMC SP assay (Figure 1). This assay is also characterized by a high fold-change of IL-2 release from stimulation with αCD28SA compared to its matched isotype (859.0). Whereas αCD3 and αCD52, while still inducing IL-2 expression, result in lower fold-changes than αCD28SA (6.2 and 3.3 for αCD3 and αCD52, respectively, Figure 2).
In the WB AQ assay (Figure 3), the level of detectable cytokines is noticeably less than in PBMC SP assay but is characterized by a greater sensitivity to stimulation by αCD52 antibody (Figure 2 and Figure 3) with mean fold-changes of IL-2, IFN-γ and IL-6 above 100.
A test antibody for which future first-in-human dosing might be anticipated not to cause any unanticipated significant increases in cytokine release compared to relevant isotype controls. Although, rather than stopping the development of a new therapeutic mAb, a positive result in a CRA should be considered as part of the risk/benefit management13. When developing a new CRA platform, the assay should be repeated using a different set of donors to ensure reproducibility of the platform. Importance should also be given to account for the variability of response between donors14, and so a well-powered experiment is recommended15. In order to understand the breadth of responses and get a complete representation of variability that could be observed in donor responses, the assay should ideally be performed with multiple individual donors in addition to assaying the therapeutic in replicate experiments.

Figure 1: Cytokine release from PBMC-SP experiment. Representative results of IFN-γ, IL-2, IL-6 and TNF-α release obtained from PBMC-SP cytokine release assays (3 independent experiments each containing 5-8 donors per experiment; n=8, n=10, n=5 [top to bottom]) after 48 h when using reference reagent antibodies. Abbreviations: PBMC = peripheral blood mononuclear cells; SP = solid phase; IFN-γ = interferon-gamma; IL-2 = interleukin 2; IL-6 = interleukin 6; TNF-α = tumour necrosis factor alpha. Please click here to view a larger version of this figure.
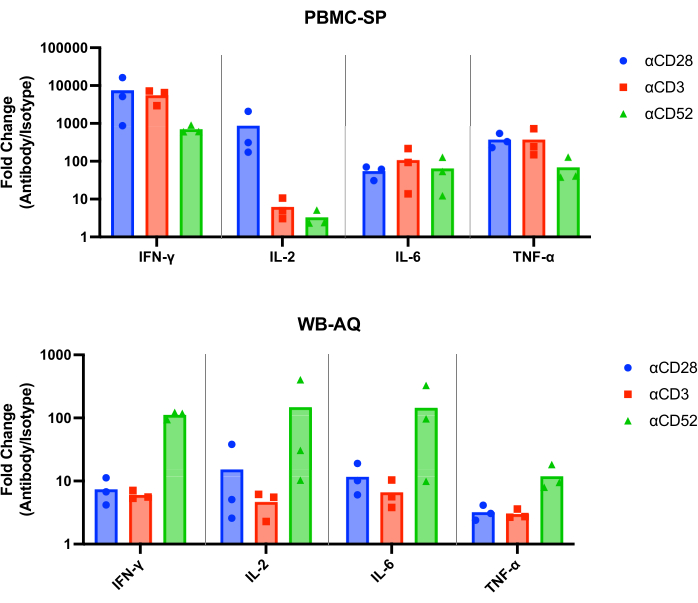
Figure 2: Cytokine fold change increase from PBMC-SP and WB-AQ experiments. Fold change of IFN-γ, IL-2, IL-6 and TNF-α release of CRS-inducing antibody reference reagents relative to their matched isotype controls, obtained from PBMC-SP and WB-AQ cytokine release assays after 48 h. Abbreviations: PBMC = peripheral blood mononuclear cells; SP = solid phase; WB = whole blood; AQ = aqueous (phase); IFN-γ = interferon-gamma; IL-2 = interleukin 2; IL-6 = interleukin 6; TNF-α = tumour necrosis factor alpha; CRS = cytokine release syndrome. Please click here to view a larger version of this figure.
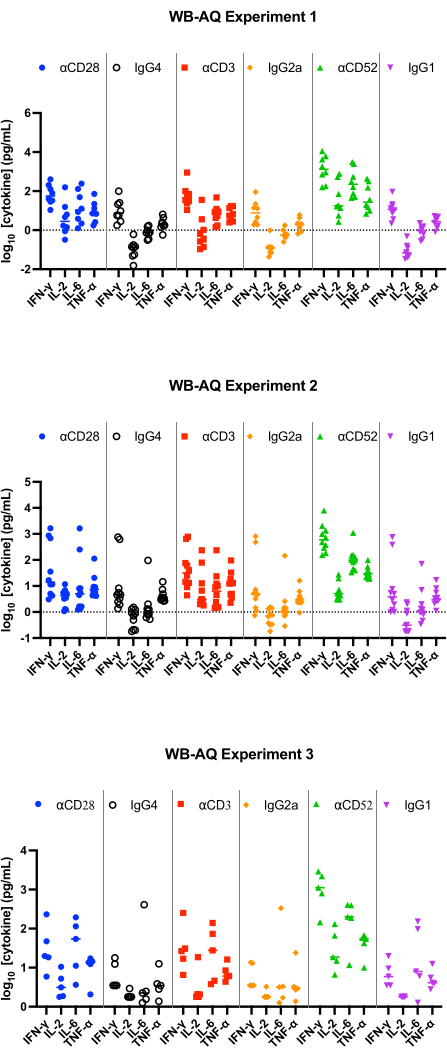
Figure 3: Cytokine release from WB-AQ experiment. Representative results of IFN-γ, IL-2, IL-6 and TNF-α release obtained from WB-AQ cytokine release assays (3 independent experiments each containing 5-8 donors per experiment; n=8, n=10, n=5 [top to bottom]) after 48 h when using reference reagent antibodies. Abbreviations: WB = whole blood; AQ = aqueous (phase); IFN-γ = interferon-gamma; IL-2 = interleukin 2; IL-6 = interleukin 6; TNF-α = tumour necrosis factor alpha. Please click here to view a larger version of this figure.
Discussion
Described here are methods of measuring cytokine release from PBMCs and WB following antibody-mediated stimulation from an antibody-coated plate or with antibody in solution, using a panel of reference reagents for positive and negative controls. Each of these assays have their own associated strengths and weaknesses. PBMC and WB assays are complementary since the proportion of various immune cells such as lymphocytes, monocytes and granulocytes are different in the two experimental matrices used for CRAs. It is interesting to observe that although a WB assay might better represent the in vivo condition as opposed to PBMC monoculture, the former platform is less predictive of T-cell mediated CRS risk from TGN1412 and OKT315; a result of glycophorin A on RBCs inhibiting IL-2 mediated T-cell expansion16. Nevertheless, the prediction of CRS risk from anti-CD52 remains intact in WB CRA, owed to the presence of neutrophils (lost during conventional density gradient methods described in step 2, used for PBMC isolation).
The format of the CRA (SP or AQ presentation) is critical for detection of specific mechanism of CRS. For example, aqueous phase presentation of the mAb to human lymphocytes17,18, employed during pre-clinical in vitro safety tests of TGN1412 failed to identify CRS risk likely due to lack of localized cell receptor clustering and engagement19 and consequent T cell activation mediated by antibody in aqueous phase. In fact, TGN1412-mediated CRS could only be accurately detected in SP format that artificially replicates Fc-gamma receptor (FcγR) cross-linking, as presented here, or by contact-dependent priming in PBMC preculture at high density and Fc interaction with CD32+ immune cells (such as B-cells20 and monocytes21).
In addition to these platforms, there are other ways of performing CRA with more complex co-culture systems. An example of an alternative CRA to those described in these methods is to co-culture PBMCs with autologous blood outgrowth endothelial cells (BOECs)22. This assay was described in 2015 as an improvement to the then conventional mixed-donor HUVEC:PBMC assay by removing the confounding tissue mismatch. It demonstrates better sensitivity to anti-CD28SA CRS than the WB assay, and also overcomes the limitation of the PBMC monoculture assay by mimicking the combination of endothelial cells and leukocytes present in vivo, but at the cost of more lengthy procedural steps requiring specialized cell culture techniques22.
Furthermore, while this protocol focuses specifically on IFN-γ, IL-2, IL-6 and TNF-α release, colleagues at MHRA have previously looked at IL-12 and others in this setting23. IL-12 production is increased by these positive control CRS antibodies, although it isn't particularly sensitive, and therefore perhaps not a great predictor of CRS in this modality. Some cytokines, such as IL-15 among others, were never tested although the 4 cytokines evaluated in our protocol provide a good indication of potential risk of CRS. Of course, depending on the modality and antibodies tested other cytokines could be assessed.
Combined, these observations highlight the importance of noting that although the use of reference reagents can help to identify CRS risk of new antibodies, care should be taken to avoid a sub-optimal CRA platform which might fail to identify CRS potential. Crucially, the predicted mechanism of action of a therapeutic, whether via its Fc region or its hypothesized action upon antigen-expressing cells, must match the biology of the assay. Therefore, while differences in the mechanism of action of the test therapeutic and reference reagents discussed in this article may pose a potential limitation in such a way that a relevant CRA for the test antibody might be incompatible with the biology of the reference reagents, the assay provides a robust, reliable platform for hazard identification. Results from several CRA formats covering various mechanisms of actions and immune cell subsets may however be necessary for optimal confidence in the safety evaluation data.
Disclosures
EM was formerly employed by Medicines and Healthcare Products Regulatory Agency (MHRA). Remaining authors have no conflicts of interest.
Acknowledgements
This work was funded by the National Institute for Biological Standards and Control. We thank Sandra Diebold for reviewing the manuscript and for helpful comments and suggestions. We are also thankful to Ka Seng Ieong for filming the video.
Materials
| Name | Company | Catalog Number | Comments |
| 1.5 ml Microcentrifuge Tubes, Natural (Sterile) | Starlab | S1615-5510 | |
| Fetal Bovine Serum, qualified, heat inactivated | ThermoFisher | 10500064 | |
| Heparinized tubes | ThermoFisher | 12967676 | |
| Heracell 150i CO2 Incubator | ThermoFisher | 16406639 | |
| MESO QuickPlex SQ 120 Human Proinflammatory Panel 1 V-PLEX kit | Meso Scale Discovery | K15049 | |
| MESO QuickPlex SQ 120MM | Meso Scale Discovery | AI1AA-0 | |
| Neubauer Improved Haemocytometer Counting Chamber | Hawksley | AS1000 | |
| Panel of lyophilized recombinant antibody controls for Cytokine Release Assays | NISBC | 19/156 | |
| PBS | ThermoFisher | 10010023 | |
| Polypropylene 96-well microtiter plate | Corning | 3879 | |
| Polystyrene 96-well microtiter plate | Corning | 3799 | |
| RPMI 1640 | ThermoFisher | 11875093 | |
| Sorvall ST 40 Centrifuge | ThermoFisher | 75004525 | |
| Sterile water | ThermoFisher | 15230162 |
References
- Suntharalingam, G., et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. The New England Journal of Medicine. 355 (10), 1018-1028 (2006).
- Hünig, T. The storm has cleared: lessons from the CD28 superagonist TGN1412 trial. Nature Reviews Immunology. 12 (5), 317-318 (2012).
- Wing, M. G., et al. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. Journal of Clinical Investigation. 98 (12), 2819-2826 (1996).
- Gaston, R. S., et al. OKT3 first-dose reaction: Association with T cell subsets and cytokine release. Kidney International. 39 (1), 141-148 (1991).
- Riechmann, L., Clark, M., Waldmann, H., Winter, G. Reshaping human antibodies for therapy. Nature. 332 (6162), 323-327 (1988).
- Kung, P., Goldstein, G., Reinherz, E. L., Schlossman, S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 206 (4416), 347-349 (1979).
- Ball, C., et al. Antibody C region influences TGN1412-like functional activity in vitro. Journal of Immunology. 189 (12), 5831-5840 (2012).
- Vessillier, S., et al. Development of the first reference antibody panel for qualification and validation of cytokine release assay platforms - Report of an international collaborative study. Cytokine: X. 2 (4), 100042 (2020).
- Findlay, L., et al. Improved in vitro methods to predict the in vivo toxicity in man of therapeutic monoclonal antibodies including TGN1412. Journal of Immunological Methods. 352 (1-2), 1-12 (2010).
- oVE Science Education Database. Science Education Database. Basic Methods in Cellular and Molecular Biology. Using a Hemacytometer to Count Cells. Journal of Visualized Experiments. , (2023).
- Lehmann, J. S., Zhao, A., Sun, B., Jiang, W., Ji, S. Multiplex Cytokine Profiling of Stimulated Mouse Splenocytes Using a Cytometric Bead-based Immunoassay Platform. Journal of Visualized Experiments. (129), e56440 (2017).
- Murthy, H., Iqbal, M., Chavez, J. C., Kharfan-Dabaja, M. A. Cytokine Release Syndrome: Current Perspectives. Immunotargets Therapy. 8, 43-52 (2019).
- Vidal, J. M., et al. In vitro cytokine release assays for predicting cytokine release syndrome: the current state-of-the-science. Report of a European Medicines Agency Workshop. Cytokine. 51 (2), 213-215 (2010).
- Grimaldi, C., et al. Cytokine release: A workshop proceedings on the state-of-the-science, current challenges and future directions. Cytokine. 85, 101-108 (2016).
- Vessillier, S., et al. Cytokine release assays for the prediction of therapeutic mAb safety in first-in man trials - Whole blood cytokine release assays are poorly predictive for TGN1412 cytokine storm. Journal of Immunological Methods. 424, 43-52 (2015).
- Chu, J. W. K., Sharom, F. J. Glycophorin A interacts with interleukin-2 and inhibits interleukin-2-dependent T-lymphocyte proliferation. Cellular Immunology. 145 (2), 223-239 (1992).
- Stebbings, R., Eastwood, D., Poole, S., Thorpe, R. After TGN1412: recent developments in cytokine release assays. Journal of Immunotoxicology. 10 (1), 75-82 (2013).
- Hanke, T. Lessons from TGN1412. Lancet. 368 (9547), 1569-1570 (2006).
- Stebbings, R., et al. #34;Cytokine storm" in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. Journal of Immunology. 179 (5), 3325-3331 (2007).
- Bartholomaeus, P., et al. Cell contact-dependent priming and Fc interaction with CD32+ immune cells contribute to the TGN1412-triggered cytokine response. Journal of Immunology. 192 (5), 2091-2098 (2014).
- Hussain, K., et al. Upregulation of FcγRIIb on monocytes is necessary to promote the superagonist activity of TGN1412. Blood. 125 (1), 102-110 (2015).
- Reed, D. M., et al. An autologous endothelial cell:peripheral blood mononuclear cell assay that detects cytokine storm responses to biologics. The FASEB Journal. 29 (6), 2595-2602 (2015).
- Eastwood, D., et al. Severity of the TGN1412 trial disaster cytokine storm correlated with IL-2 release. British Journal of Clinical Pharmacology. 76 (2), 299-315 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved