5.3 : Acid and Bases: Ka, pKa, and Relative Strengths
This lesson delves into a critical aspect of the relative strengths of acids and bases. The strength of an acid is evaluated by the acid dissociation into its conjugate base and a hydronium ion in water. The complete dissociation of a strong acid is confirmed with a very high concentration of hydronium ions. As a result, an incomplete dissociation process affirms a weak acid. Therefore, the equilibrium is in the forward direction for strong acids and backward for weak acids in these reactions.
Accordingly, the acid strength is defined by the concentration of undissociated acid molecules and hydronium ions. While the weak acid can be estimated via the equilibrium constant (Keq), it is constant for a dilute solution, and the change in water concentration is negligible. This observation leads to a modified equilibrium constant known as the acidity constant or dissociation constant, Ka. To define the acidity constant that is a scale of acidity, consider the generic acid-base reaction:
Figure 1: Dissociation of a generic acid in water
Here, HA denotes the generic acid, and A− denotes its conjugate base. The following expression represents the acidity constant for this reaction.
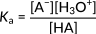
Figure 2: Acidity constant for a generic acid dissociation
This relationship focuses on the concentration of hydronium ions in the numerator. Accordingly, an increase in these ions leads to an increasing acidity constant and a stronger acid. In organic acids, typically, the magnitude of Ka is spread across several orders. Hence, the strength of different acids is expressed in terms of pKa values, calculated as the negative logarithm of Ka:
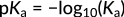
Figure 3: Expression of pKa
Here, the minus sign indicates the inverse relationship between the pKa value and acidity. As elucidated with benzoic acid versus hydrobromic acid, a higher pKa value equals a lower Ka value, which indicates a weaker acid.
By extension of the above principle, the pKa values can also establish the strength of a base. Since the dissociation of a base forms a conjugate acid, a stronger conjugate acid corresponds to a weaker base. For instance, consider methanol versus ethylamine. The conjugate acid of methanol with a pKa value of −3.8 is more acidic than the conjugate acid of ethylamine with a pKa value of 10.6. Hence, methanol is a weaker base than ethylamine.
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。






