3.13 : Introduction to Enzyme Kinetics
Enzyme kinetics studies the rates of biochemical reactions. Scientists monitor the reaction rates for a particular enzymatic reaction at various substrate concentrations. Additional trials with inhibitors or other molecules that affect the reaction rate may also be performed.
The experimenter can then plot the initial reaction rate or velocity (Vo) of a given trial against the substrate concentration ([S]) to obtain a graph of the reaction properties. For many enzymatic reactions involving a single substrate, this data fits the Michaelis-Menten equation, an equation derived by Leonor Michaelis and Maud Menten.
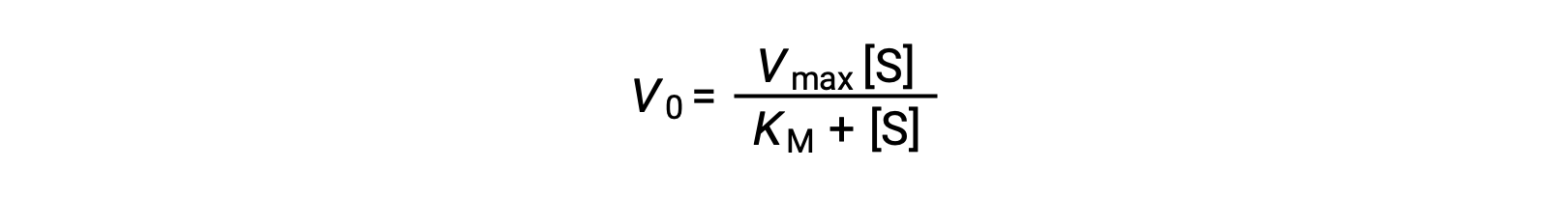
The equation estimates the maximum velocity (Vmax) and the Michaelis constant (KM) for the enzyme being studied and is based on the following assumptions:
- No product is present at the start of the reaction.
- The rate of enzyme-substrate complex formation equals the rate of dissociation and breakdown into products.
- The enzyme concentration is minimal compared to the substrate concentration.
- Only the initial reaction rates are measured.
- The enzyme is present either in the free form or in the enzyme-substrate complex.
Different rearrangements of the Michaelis-Menten equation, such as the Lineweaver-Burke, Eadie-Hofsteot, and Hanes-Woolf plots, are alternate ways to graph kinetic parameters. The Lineweaver-Burke or double reciprocal plot is often used to estimate the KM and the Vmax. The plot uses the reciprocals values of the x and y-axis from the Michaelis-Menten plot. Mathematically, the y-intercept equals 1/Vmax, and the x-intercept equals −1/KM.
The Lineweaver-Burke plot can be used to visually differentiate between inhibitor types – competitive, non-competitive, and uncompetitive. Different rearrangements of the Michaelis-Menten equation, such as the Eadie-Hofstee and Hanes-Woolf plots, are also used to determine kinetic parameters.
来自章节 3:

Now Playing
3.13 : Introduction to Enzyme Kinetics
Energy and Catalysis
19.7K Views

3.1 : 热力学第一定律
Energy and Catalysis
5.5K Views

3.2 : 热力学第二定律
Energy and Catalysis
5.2K Views

3.3 : 细胞内焓
Energy and Catalysis
5.8K Views

3.4 : Cell 内的熵
Energy and Catalysis
10.4K Views

3.5 : 自由能源简介
Energy and Catalysis
8.2K Views

3.6 : 细胞内的内能和能能反应
Energy and Catalysis
14.8K Views

3.7 : 平衡结合常数和结合强度
Energy and Catalysis
9.0K Views

3.8 : 自由能和平衡
Energy and Catalysis
6.0K Views

3.9 : 单元中的非平衡
Energy and Catalysis
4.2K Views

3.10 : 有机分子的氧化和还原
Energy and Catalysis
6.2K Views

3.11 : 酶简介
Energy and Catalysis
17.2K Views

3.12 : 酶和活化能
Energy and Catalysis
11.7K Views

3.14 : 周转次数和催化效率
Energy and Catalysis
9.9K Views

3.15 : 催化完美的酶
Energy and Catalysis
3.9K Views
See More
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。