需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Method Article
人外周血 NK 细胞活性评估流基于流式细胞仪检测细胞毒性检测
Erratum Notice
摘要
示了流基于流式细胞仪的方法定量地确定人类自然杀手细胞的细胞毒活性。
摘要
先天免疫系统内效应器淋巴细胞称为自然杀伤 (NK) 细胞在宿主防御对异常细胞,专门消除肿瘤和病毒感染细胞发挥了重要的作用。大约 30 已知的单基因缺陷,一大批其他的病理状况,导致功能或经典 NK 细胞缺陷,表现在减少或缺失细胞毒活性。历史上,还用放射性方法,它是繁琐、 昂贵和有潜在危险的方法研究了细胞毒性。这篇文章描述了一个精简、 临床适用流流式细胞仪基于方法量化 NK 细胞杀伤活性。这种测定方法,外周血单个核细胞 (外周血) 或纯化的 NK 细胞制剂在不同比例共同孵育与已知为 NK 细胞介导的细胞毒性 (NKCC) 对敏感目标肿瘤细胞系。靶细胞是预先标记荧光的荧光染料,使他们从效应器细胞 (NK 细胞) 的歧视。后潜伏期,杀死的靶细胞核酸染色,具体地渗透到死细胞由标识。此方法是服从既诊断研究和应用,得益于多参数流式细胞的能力,有好处,有可能使 NK 细胞表型和功能的更深入的分析。
引言
自然杀伤 (NK) 细胞是人类先天淋巴细胞批判地参与消除病毒感染的细胞,转化的细胞和其他致病性威胁1,2的复杂的子集。NK 细胞裂解颗粒房子像穿孔素和 granzymes 细胞毒性蛋白质。激活后,NK 细胞形成复杂的互动关系,与他们的目标称为免疫突触,藉以本地释放这些细胞毒性分子,造成的直接目标细胞裂解和细胞凋亡与细胞因子和趋化因子的释放,并最终在诱导炎症状态1,,34。
NK 细胞活化涉及复杂的字符串的激活和抑制相互作用 NK 细胞受体与配体形成严格监管的系统的靶细胞表面表达。NK 细胞活化的研究最机制之一是"缺少自我"。事实上,缺乏检测类我主要组织相容性复合物 (MHC) 上或人类白细胞抗原 (HLA) 分子的感染或转化细胞触发器 NK 细胞毒性。肿瘤和病毒感染的细胞普遍下调这些抗原逃避 T 细胞介导的免疫功能,从而成为初级 NK 细胞目标1,,34。
NK 细胞功能的评估主要分为肥大或细胞毒性检测方法。然而,脱颗粒含量测定,如 CD107a,肥大关联标记流流式细胞术检测仅仅是功能的指示性的 NK 细胞活化而不是功能的其最终,直接杀死目标细胞5,,67,8的。因此,这种限制已经提请调查人员细胞毒性试验作为一种更生动、 更直接的替代。
长期的"金本位"评估细胞介导的细胞毒活性的 T 和 NK 细胞是铬释放检测 (CRA)。CRA 涉及放射性标记的靶细胞与51Cr 和效应器细胞共同孵育他们。这种测定方法被沉浸在那细胞裂解结果中释放的蛋白质绑定51Cr 到上清液,可以通过伽玛计数测量的原理。这种测定方法,同时有效,是有问题的各种原因: 材料成本高、 处理和处置放射性51Cr、 51Cr,自发释放和困难标准化-使它完全不切实际9,10。
大量的非放射性检测,涉及荧光标记、 酶释放和甚至生物发光,已经得到发展,作为 CRA 11,,1213,14的替代品。在这里,我们可以描述流测量的 NK 细胞杀伤活性对 k562 细胞的靶细胞,是简单、 灵敏,基于流式细胞仪的方法。K562 细胞是人类 erythroleukemic 细胞线与 HLA 类表达减少第一和配体的活化 NK 受体,这使得它们特别容易受到 NK 细胞介导的细胞毒性15高度的表达。在这种测定方法,K562 细胞前带有羧基荧光素二乙酸琥珀酰亚胺酯 (CFSE) 和共培养在各种比率与任一外周血单个核细胞 (外周血) 或纯化 NK 细胞1。CFSE 是稳定、 蛋白结合的荧光染料,允许歧视的靶细胞效应器 NK 细胞16,17日。共同孵育后, 核酸染色,具体渗透膜的死细胞,用于标识被杀死的靶细胞 (见材料表)。样品上流式细胞仪来确定死 (即,染色 +) 的百分比,得出 CFSE + 靶细胞。
这种测定方法可以用作常规的诊断筛查,为单基因缺陷影响 NK 细胞隔间,其中有大约 30 已知的缺陷导致功能或经典 NK 细胞缺乏症,并为原发性或继发性噬血细胞综合征。它也是用于调查患者反复发作,严重的疱疹病毒感染,评价免疫重建造血细胞移植或邮政免疫调节治疗18,,1920后, 为基本研究应用程序宿主 NK 细胞活性。
Access restricted. Please log in or start a trial to view this content.
研究方案
Samples were collected according to the ethical guidelines established by the UCLA Human Research Protection Program and IRB approved.
1. Preparation of reagents
NOTE: Unless otherwise stated, all reagents should be allowed to equilibrate at room temperature prior to use. All reagents must remain sterile.
- Prepare a 2x working solution of Tween-20 (i.e., 0.2%) by adding 10 µL of Tween-20 solution to 5 mL of phosphate-buffered saline (PBS) without calcium and magnesium with a p20 pipette.
- Given the high viscosity of Tween-20, take the following steps to ensure accuracy: collect slowly to avoid bubble formation, do not submerge the entire tip to avoid carry-over, and pipette up and down several times in PBS to wash out all Tween-20 from the tip.
- Add 2 µL of IL-2 stock solution (2.1E6 IU/mL) to 198 µL of complete media (i.e., 1:100 dilution), which is RPMI with 1% penicillin-streptomycin and 10% fetal bovine serum (FBS), then proceed to an additional 1:100 dilution by adding 2 µL of IL-2 intermediate solution to 198 µL of complete media to prepare a 7x working solution (210 IU/mL).
- Add 2 µL of CFSE stock solution (5 mM) to 500 µL of plain media (RPMI). Vortex, spin down to remove drops from the cap, then proceed with an additional 1:20 dilution by adding 50 µL of the CFSE intermediate solution to 950 µL of plain RPMI to obtain a 2x working solution of 1 µM. Vortex well and keep protected from light.
2. Isolation of PBMCs as effector cells
NOTE: This assay has been validated for effective use with total PBMCs from healthy controls. However, it is recommended that NK cell content be verified with each PBMC preparation (Figure 1). Also, the volume of whole blood for collection is based on the frequency of NK cells in peripheral whole blood and this may vary from person to person, particularly between healthy donors and patients.
- Collect a minimum of 4 mL of human whole blood in sodium or lithium heparinized blood collection tubes. Store blood samples at room temperature upon collection and use within 30 h of collection.
- Dilute whole blood with 4 mL of PBS (i.e. equal volume of PBS to whole blood).
- Add 4 mL of density gradient solution (see table of materials) to a 15-mL conical tube. Carefully overlay diluted whole blood over the density gradient solution by tilting the tube.
- Centrifuge at 650 x g for 24 min with the brake off.
- Carefully collect the layer of mononuclear cells, which is the thin white layer below the top layer of plasma and platelets, into a new 15-mL conical tube. Bring the volume to 15 mL with PBS.
- Centrifuge at 450 x g for 10 min with the brake on. Aspirate the supernatant and resuspend cells in 0.5 mL of complete media.
- Use an automated cell counter or hemocytometer to count the cells, and adjust the PBMC concentration to 5x106 cells/mL with complete media.
- Check the NK cell content by flow cytometry (see step 4).
- Start the assay within 30 min. Keep cells at room temperature for up to 10 min, or place in a humidified 5% CO2 incubator at 37 °C until ready for use.
3. Isolation of NK cells as effector cells
NOTE: This portion of the protocol is an alternative to using total PBMCs as effector cells. The typical yield from 4 mL of whole blood from a healthy individual is approximately 4x105 NK cells, or approximately 5-15% of PBMCs, though this frequency varies between donors 21. It is recommended that a purity check be performed after the isolation.
- Collect a minimum of 4 mL of human whole blood in sodium or lithium heparinized blood collection tubes. Take 50 µL of whole blood to stain as a pre-enrichment sample.
- Add the enrichment cocktail to blood samples at 50 µL/mL (see table of materials). Mix well and incubate for 10 min at room temperature.
- Add an equal volume of PBS + 2% FBS to dilute the sample and gently mix well.
- Add 4 mL of density gradient solution to a 15-mL conical tube. Carefully overlay the diluted sample on top of the density gradient medium.
- Centrifuge at 1,200 x g for 10 min with the brake off.
- Carefully collect the layer of enriched cells into a new 15-mL conical tube. Wash the cells by bringing the volume up to 50 mL with PBS + 2% FBS.
- Centrifuge at 300 x g for 10 min. Discard the supernatant and repeat the wash.
- Aspirate the supernatant and resuspend cells in 0.5 mL of complete media.
- Using an automated cell counter or hemocytometer, count the cells and adjust the concentration to 5x105 cells/mL. Take 50 µL of purified cell suspension to stain as a post-enrichment sample.
- Keep cells at room temperature for up to 10 min, or place in a humidified 5% CO2 incubator at 37 °C until ready for use.
4. Immunophenotyping for assessment of NK cell content
- Using 50 µL total isolated PBMCs, pre-enrichment whole blood, or purified NK cells, stain with anti-CD56 fluorochrome-conjugated antibody as an NK cell marker. Markers for other cell types and lineages (i.e. CD3+ T cells, CD19+ B cells) may also be included to assess the origin of contamination, if any.
- Incubate for 20 min at 4 °C, and wash by adding 2 mL of PBS + 2% FBS.
- Centrifuge at 450 x g for 5 min.
- For PBMCs or NK cells, aspirate supernatant and resuspend cells in 100 µL of PBS + 2% FBS.
- For whole blood samples, perform red blood cell (RBC) lysis:
- After centrifugation, aspirate the supernatant and resuspend in 2 mL red blood cell lysis buffer (31.75 g of ammonium chloride + 4.16 g of Tris base + 6.65 g of EDTA, dissolved in 5000 mL of deionized water).
- Incubate for 10 min at room temperature.
- Repeat wash twice, aspirate the supernatant, and resuspend lysed sample in 100 µL of PBS + 2% FBS.
NOTE: RBC lysis may also be performed prior to staining. - Proceed with flow cytometric analysis.
5. Thawing, culturing, and harvesting of K562 target cells
- Aliquot 10 mL of complete media into a 15-mL conical tube and allow to warm in a 37 °C water bath. For the thawing and culturing of K562 cells, use only media pre-warmed to 37 °C for all steps.
- Place one vial of frozen K562 cells in the 37 °C water bath until most of it is thawed.
- Transfer the semi-thawed K562 cells to the complete media, pre-warmed to 37 °C, in the 15-mL conical tube. Wash the inside of the cryogenic vial with some pre-warmed media, and add the wash to the 15-mL tube.
- Centrifuge at 140 x g for 7 min with the brake on. Remove the supernatant and resuspend the pellet in 1 mL of complete media.
- Aliquot an appropriate amount of cell suspension to count cells using an automated cell counter or hemocytometer.
- Adjust the concentration to 1x105-2x105 cells/mL and culture in a humidified 5% CO2 incubator at 37 °C for at least 2 days before use. Maintain the culture at this density range for no longer than 3 months, then thaw a new aliquot. Never exceed a cell concentration of 1x106 cells/mL in culture.
- For use in the assay, mix the K562 cell culture well with a 10-mL serological pipette and remove an aliquot to count and assess cell viability using an automated cell counter or hemocytometer and Trypan Blue staining.
- If the viability is less than 85%, proceed with the following dead cell removal steps.
- Add 15 mL of density gradient solution to 50-mL conical tubes, as needed. Collect all cells from culture and slowly overlay the cell suspension over the density gradient solution by tilting the tube.
- Centrifuge at 650 x gfor 24 min with brake off. Carefully collect the layer of live cells into a new 15-mL conical tube. Bring the volume to 50 mL with PBS.
- Centrifuge at 450 x g for 10 min with the brake on. Remove the supernatant and resuspend the pellet in 1 mL of complete media. Count the cells and check cell viability (as in 5.7).
- Culture in a humidified 5% CO2 incubator at 37 °C for at least 2 days before use.
- If the viability is less than 85%, proceed with the following dead cell removal steps.
- Collect 5x105 K562 target cells and centrifuge at 140 x g for 7 min. Aspirate the supernatant and resuspend the cell pellet in 500 µL in media with 1% FBS.
6. Labeling of K562 target cells
NOTE: Make sure the K562 cells are well resuspended before adding the CFSE working solution.
- Pipette 500 µL of the CFSE working solution to the 500 µL of K562 cells for a final concentration of 0.5 µM. Using the same 1000-1250 µL tip, mix immediately by gently pipetting up and down 3-5 times.
- Incubate in the 37 °C water bath or in a humidified 5% CO2 incubator at 37 °C for 10 min in the dark.
NOTE: Since the temperature equilibration lag time is longer in the incubator compared to the water bath, incubation time might need to be adjusted especially for volumes > 1 mL. - Quench the labeling reaction with 10 mL of complete media for 10 min in the dark at room temperature.
- Centrifuge the labeled K562 cells at 140 x g for 7 min. Remove the supernatant and resuspend in 1 mL of complete media.
- Count the cells using an automated cell counter or hemocytometer and bring the concentration to 1x105 cells/mL in complete media.
- Place cells in a humidified 5% CO2 incubator at 37 °C until ready for use. Cells can be kept at room temperature for up to 10 min, or in in a humidified CO2 incubator at 37 °C until ready for use. Do not refrigerate.
7. Plating
NOTE: If NK cells are limiting, the concentration of both K562 and NK cells can be halved, so that half of the cells are plated in the assay. The assay yields comparable results with half the amount of cells (as long as the ratios are kept consistent).
- If using total PBMCs, label a 96-well, U-bottom, tissue culture-treated plate for the following conditions per well: (1) Effector (E) : Target (T) = 50 : 1 + IL-2 - positive control for NK cell cytotoxicity; (2) E : T = 50 : 1; (3) E : T = 25 : 1; (4) E : T = 12.5 : 1; (5) E : T = 6.25 : 1; (6) T - target cells only - negative control for K562 death; (7) E - effector cells only; (8) T + Tween - Positive control for K562 death
- If using purified NK cells, label a 96-well, U-bottom, tissue culture treated plate for the following conditions per well: (1) Effector (E) : Target (T) = 5: 1 + IL-2 - positive control for NK cell cytotoxicity; (2) E : T = 5 : 1; (3) E : T = 2.5 : 1; (4) E : T = 1.25 : 1; (5) E : T = 0.625: 1; (6) T - target cells only - negative control for K562 death; (7) E - effector cells only; (8) T + Tween - positive control for K562 death
- Add 100 µL of complete media to conditions 3, 4, 5, 6, 7. For this and all subsequent steps, (recommended) use a multichannel pipette.
- Add 100 µL of effector cells (PBMCs at 5x106 cells/mL or purified NK cells at 5x105 cells/mL) to conditions 1, 2, 3. Add effector cells for conditions 4-7 by serial dilution. Do not change tips from condition to condition.
- Mix condition 3, take 100 µL, and transfer to condition 4.
- Mix condition 4, take 100 µL, and transfer to condition 5.
- Mix condition 5, take 100 µL, and transfer to condition 7; Condition 6 does not have effector cells.
- Add 100 µL of prepared 2x 0.2% Tween working solution to condition 8 (final concentration: 0.1% Tween-20); condition 8 does not have effector cells.
- Add 30 µL of IL-2 working solution to condition 1. IL-2 should be added to the effector cell suspension prior to the addition of target cell suspension (final concentration: 27 IU/mL).
- Add 100 µL of target cells (labeled K562 cells at 1x105 cells/mL) to conditions 1, 2, 3, 4, 5, 6, 8. Condition 7 does not have target cells.
- Mix all wells with a multichannel pipette and centrifuge the plate at 120 x g for 2 min.
- Incubate in a humidified CO2 incubator at 37 °C for 4 h. At the end of the incubation, place the plate on ice and proceed to viability staining within 30 min.
8. Staining for viability and acquisition
- Prepare dead cell stain (see table of materials) by adding 3 µL of the 5 µM stock solution to 600 µL of PBS.
- Add 50 µL of diluted dead cell stain to each well for a final volume of 250 µL and a final concentration of 0.005 µM. Mix well with a multichannel pipette.
- Proceed with flow cytometric analysis. Using a flow cytometer, collect at least 1,000 events in the target cell gate (Figure 2) for statistically meaningful results.
Access restricted. Please log in or start a trial to view this content.
结果
在设置前检测,强烈建议 NK 细胞内容会在选择的效应器人口评估。图 1显示了典型 CD56 沾色 (浅蓝色) 之前和之后 (红色) NK 细胞富集。NK 细胞组成达 15%的外周血和浓缩后应至少 80%的纯。
流式细胞术分析这种测定方法中的涉及的两个参数的检测: CFSE,检出相同的通道作为 FITC;和死细胞染色,在相同?...
Access restricted. Please log in or start a trial to view this content.
讨论
这里介绍的方法提供了一个简单和具有成本效益的替代传统51铬释放检测评估 NK 细胞杀伤活性。此方法是灵敏、 重现性好,比以前的标准方法,像 cra 法案,耗时少,可以用于临床和研究应用程序。
同时测定工程与总外周血和浓缩的 NK 细胞,而无需使用外周血净化细胞群体的选项是极大的好处与小卷收集的血或少或质量差细胞来自病人的样本打交道时。这种测定方?...
Access restricted. Please log in or start a trial to view this content.
披露声明
作者宣称没有的财务上的利益冲突。
致谢
我们要感谢吉尔纳西索,加州大学洛杉矶分校免疫遗传学中心,她求助与手稿的准备。
Access restricted. Please log in or start a trial to view this content.
材料
| Name | Company | Catalog Number | Comments |
| Phosphate-buffered Saline (1x, w/o Ca2+ and Mg2+) | Corning (Cellgro) | 21-040-CM | |
| Ficoll-Paque PLUS | GE Healthcare | 17-1440-02 | |
| Tween-20 | Sigma | BP337-100 | |
| RPMI 1640 Media | Corning (Cellgro) | 10-040-CV | |
| Heat-inactivated Fetal Bovine Serum | Omega Scientific | FB-02 | |
| Penicillin Streptomycin | Life Technologies | 15140-163 | Stock solution at 10,000 U/mL |
| IL-2 | R&D Systems | 202-IL-050 | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA with BSA as a carrier protein. Reconstitute with 500 ul at 100 μg/mL in sterile 100 mM Acetic Acid containing at least 0.1% bovine serum albumin (2.1x10E6 IU/ml) |
| K562 Cells | ATCC | CCL-243 | Cancer cell line |
| T-75 cell culture flasks | Corning | 431464 | |
| CFSE cell proliferation kit | Life Technologies (CellTrace) | C34554 | Reconstitute I vial with 18 ul DMSO to prepare a 5mM stock solution. Do not freeze/thaw. |
| Sytox Red | Life Technologies | S34859 | Stock solution is provided at 5 μM in 1 mL DMSO. The DMSO solution may be subjected to multiple freeze-thaw cycles without reagent degradation. |
| Sodium/lithium heparin blood collection tubes | BD | 02-687-95 | |
| U-bottom 96-well plate | Corning | CLS3897 | |
| Serological pipettes | BD Falcon | ||
| Polystyrene round-bottom tubes (5mL) | BD Falcon | 14959-5 | |
| 50 mL polypropylene conical tube | BD Falcon | 352070 | |
| 15 mL polypropylene conical tube | BD Falcon | 352097 | |
| Reagent reservoir | USA Scientific | 2321-2230 | |
| Human NK cell enrichment cocktail | StemCell Technologies (RosetteSep) | 15065 |
参考文献
- Iannello, A., Debbeche, O., Samarani, S., Ahmad, A. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J Leukoc Biol. 84 (1), 1-26 (2008).
- Caligiuri, M. A. Human natural killer cells. Blood. 112 (3), 461-469 (2008).
- Topham, N. J., Hewitt, E. W. Natural killer cell cytotoxicity: how do they pull the trigger? Immunology. 128 (1), 7-15 (2009).
- Warren, H. S., Smyth, M. J. NK cells and apoptosis. Immunol Cell Biol. 77 (1), 64-75 (1999).
- Tognarelli, S., Jacobs, B., Staiger, N., Ullrich, E. Flow Cytometry-based Assay for the Monitoring of NK Cell Functions. J Vis Exp. (116), (2016).
- Somanchi, S. S., McCulley, K. J., Somanchi, A., Chan, L. L., Lee, D. A. A Novel Method for Assessment of Natural Killer Cell Cytotoxicity Using Image Cytometry. PLoS One. 10 (10), e0141074(2015).
- Alter, G., Malenfant, J. M., Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 294 (1-2), 15-22 (2004).
- Atkinson, E. A., Gerrard, J. M., Hildes, G. E., Greenberg, A. H. Studies of the mechanism of natural killer (NK) degranulation and cytotoxicity. J Leukoc Biol. 47 (1), 39-48 (1990).
- Kim, G. G., Donnenberg, V. S., Donnenberg, A. D., Gooding, W., Whiteside, T. L. A novel multiparametric flow cytometry-based cytotoxicity assay simultaneously immunophenotypes effector cells: comparisons to a 4 h 51Cr-release assay. J Immunol Methods. 325 (1-2), 51-66 (2007).
- Kane, K. L., Ashton, F. A., Schmitz, J. L., Folds, J. D. Determination of natural killer cell function by flow cytometry. Clin Diagn Lab Immunol. 3 (3), 295-300 (1996).
- Jang, Y. Y., et al. An improved flow cytometry-based natural killer cytotoxicity assay involving calcein AM staining of effector cells. Ann Clin Lab Sci. 42 (1), 42-49 (2012).
- Korzeniewski, C., Callewaert, D. M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 64 (3), 313-320 (1983).
- Karimi, M. A., et al. Measuring cytotoxicity by bioluminescence imaging outperforms the standard chromium-51 release assay. PLoS One. 9 (2), e89357(2014).
- Oppenheim, D. E., et al. Glyco-engineered anti-EGFR mAb elicits ADCC by NK cells from colorectal cancer patients irrespective of chemotherapy. Br J Cancer. 110 (5), 1221-1227 (2014).
- West, W. H., Cannon, G. B., Kay, H. D., Bonnard, G. D., Herberman, R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 118 (1), 355-361 (1977).
- Jedema, I., van der Werff, N. M., Barge, R. M., Willemze, R., Falkenburg, J. H. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood. 103 (7), 2677-2682 (2004).
- Lecoeur, H., Fevrier, M., Garcia, S., Riviere, Y., Gougeon, M. L. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J Immunol Methods. 253 (1-2), 177-187 (2001).
- Carotta, S. Targeting NK Cells for Anticancer Immunotherapy: Clinical and Preclinical Approaches. Front Immunol. 7, 152(2016).
- Mandal, A., Viswanathan, C. Natural killer cells: In health and disease. Hematol Oncol Stem Cell Ther. 8 (2), 47-55 (2015).
- Rezvani, K., Rouce, R. H. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front Immunol. 6, 578(2015).
- Angelo, L. S., et al. Practical NK cell phenotyping and variability in healthy adults. Immunol Res. 62 (3), 341-356 (2015).
- Zons, P., et al. Comparison of europium and chromium release assays: cytotoxicity in healthy individuals and patients with cervical carcinoma. Clin Diagn Lab Immunol. 4 (2), 202-207 (1997).
- Yovel, G., Shakhar, K., Ben-Eliyahu, S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. 81 (2), 254-262 (2001).
- Laue, T., et al. Altered NK cell function in obese healthy humans. BMC Obes. 2, 1(2015).
- Hazeldine, J., Lord, J. M. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res Rev. 12 (4), 1069-1078 (2013).
Access restricted. Please log in or start a trial to view this content.
Erratum
Formal Correction: Erratum: A Flow Cytometry-Based Cytotoxicity Assay for the Assessment of Human NK Cell Activity
Posted by JoVE Editors on 9/10/2017. Citeable Link.
An erratum was issued for: A Flow Cytometry-Based Cytotoxicity Assay for the Assessment of Human NK Cell Activity. Figure 4 has been corrected to show background-corrected data.
Figure 4 was updated from:
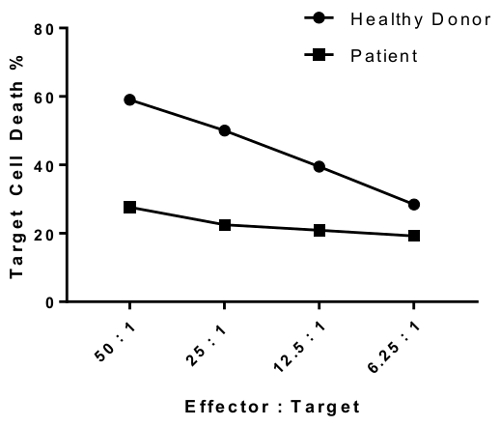
to:
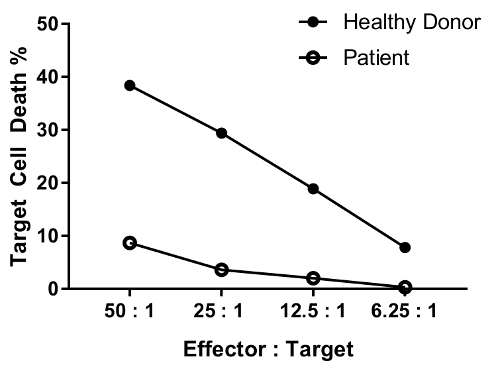
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。