Se requiere una suscripción a JoVE para ver este contenido. Inicie sesión o comience su prueba gratuita.
Method Article
Un análisis de citotoxicidad basados en la citometría de flujo para la evaluación de la actividad de las células NK humanas
En este artículo
Erratum Notice
Resumen
Aquí se muestra un método basado en la citometría de flujo para determinar cuantitativamente la actividad citotóxica de las células de asesino naturales humanas.
Resumen
Dentro del sistema inmune innato, los linfocitos efectores conocidos como células de naturales del asesinas (NK) juegan un papel esencial en la defensa del huésped contra las células aberrantes, especialmente eliminando tumoral y viralmente infectadas las células. Aproximadamente 30 defectos monogénicas conocidos, junto con una serie de otras condiciones patológicas, deficiencia funcional o clásico NK célula, que se manifiesta en reducción o ausencia de actividad citotóxica. Históricamente, se ha investigado la citotoxicidad con métodos radiactivos, que son engorrosos, costosos y potencialmente peligrosos. Este artículo describe un método basado en la citometría de flujo aerodinámico, clínicamente aplicable para cuantificar la actividad citotóxica de células NK. En este ensayo, las células mononucleares de sangre periférica (PBMCs) o preparaciones de células NK purificadas son co se incubó a relaciones diferentes con una línea de células de tumor objetivo sabida que es sensible a la citotoxicidad mediada por células NK (NKCC). Las células diana son las marcadas con un colorante fluorescente para permitir la discriminación de las células efectoras (células NK). Después del período de incubación, las células diana mataron son identificadas por una mancha de ácido nucleico, que impregna específicamente células muertas. Este método es susceptible de diagnóstico y aplicaciones de investigación y, gracias a las capacidades de múltiples parámetros de la citometría de flujo, tiene la ventaja añadida de que potencialmente permite un análisis más profundo del fenotipo de la célula NK y la función.
Introducción
Células de naturales del asesinas (NK) son un subconjunto sofisticado de linfocitos innatos humanos críticamente implicados en la eliminación de las células viralmente infectadas, las células transformadas y otras amenazas patógenas 1,2. Casa de gránulos líticos de la célula NK proteínas citotóxicas, como perforin y granzymes. Tras la activación, las células NK forman una interacción compleja con sus objetivos conocidos como sinapsis inmunológica, por el que estas moléculas citolíticas se liberan localmente, dando por resultado la lisis de la célula objetivo directo y apoptosis, junto con liberación de citoquinas y quimioquinas y en última instancia en la inducción de un inflamatorio estado 1,3,4.
Activación de la célula NK involucra una compleja cadena de activación y las interacciones inhibitorias entre NK célula receptores y ligandos expresadas en la superficie de las células de la blanco, formando un sistema fuertemente regulado. Uno de los mecanismos más estudiados de la activación de células NK es la "falta". De hecho, falta de detección de clase en que mayor complejo de histocompatibilidad (MHC), o moléculas de leucocito humano antígeno (HLA), infectados o transformado citotoxicidad celular de células desencadenantes NK. Tumorales y células infectadas por virus generalmente regular a la baja estos antígenos para escapar de la inmunidad mediada por células T, convirtiéndose en principal NK célula objetivos 1,3,4.
Evaluación de la función de la célula NK se categoriza principalmente en degranulación o citotoxicidad ensayos. Sin embargo, ensayos de degranulación, como detección de citometría de flujo del degranulation asociados marcador CD107a, sólo son indicativos de la activación de células NK y no de su función final, la muerte directa de las células objetivo 5,6,7,8. Por lo tanto, esta limitación ha dibujado los investigadores ensayos de citotoxicidad como alternativa más directa y más revelador.
El largo plazo "gold standard" para evaluar la actividad citotóxica mediada por células de las células T y NK es el ensayo de liberación de cromo (CRA). CRA implica radiactivo etiquetado de células diana con 51Cr y co los incuban con las células efectoras. Este ensayo se empapa en el principio que lisis celular resulta en la liberación de proteína determinada 51Cr en el sobrenadante, que puede ser medido contando gamma. Este ensayo, al mismo tiempo eficaz, es problemático para una variedad de razones: altos costos de material, manejo y disposición de radiactivo 51Cr, lanzamiento espontáneo de 51Cr y difícil estandarización - lo que en conjunto práctico 9,10.
Un número de ensayos no radiactivo, con etiquetado fluorescente, liberación de enzima e incluso bioluminiscencia, desde entonces se han desarrollado como alternativas a la CRA 11,12,13,14. Aquí describimos un método basado en la citometría de flujo para medición de NK célula actividad citotóxica en las células K562 objetivo simple, sensible y reproducible. Células K562 son una células erythroleukemic humana la línea con menor expresión de HLA clase I y una mayor expresión de ligandos para receptores NK activatory, que los hace particularmente susceptibles a NK Citotoxicidad mediada por células 15. En este ensayo, las células K562 están marcadas previamente con carboxyfluorescein diacetato succinimidyl éster (CFSE) y cultivan conjuntamente en diferentes proporciones con cualquiera células mononucleares de sangre periférica (PBMCs) o purificaron de células NK 1. CFSE es un colorante fluorescente estable, proteínas de Unión que permite la discriminación de las células de la blanco del efectoras NK células 16,17. Después de la incubación Co, una mancha de ácido nucleico, específicamente impregnando la membrana de las células muertas, se utiliza para identificar las células de la blanco matado (véase tabla de materiales). Las muestras entonces se adquieren en un citómetro de flujo para determinar el porcentaje de muertos (es decir, mancha +) CFSE + células diana.
Este ensayo puede utilizarse como una investigación rutinaria de diagnóstico para defectos monogénicas, que afectan el compartimiento de células NK, que son aproximadamente 30 defectos conocidos que causan deficiencia de células NK ya sea funcional o clásica, y para el lymphohistiocytosis hemophagocytic primario o secundario. También es útil para investigar la actividad de células NK en pacientes con infecciones virales herpes recurrente, grave, para evaluar la reconstitución inmune tras el trasplante de células hematopoyéticas o post inmunomoduladores terapia 18,19,20y de una serie de aplicaciones de la investigación básica.
Access restricted. Please log in or start a trial to view this content.
Protocolo
Samples were collected according to the ethical guidelines established by the UCLA Human Research Protection Program and IRB approved.
1. Preparation of reagents
NOTE: Unless otherwise stated, all reagents should be allowed to equilibrate at room temperature prior to use. All reagents must remain sterile.
- Prepare a 2x working solution of Tween-20 (i.e., 0.2%) by adding 10 µL of Tween-20 solution to 5 mL of phosphate-buffered saline (PBS) without calcium and magnesium with a p20 pipette.
- Given the high viscosity of Tween-20, take the following steps to ensure accuracy: collect slowly to avoid bubble formation, do not submerge the entire tip to avoid carry-over, and pipette up and down several times in PBS to wash out all Tween-20 from the tip.
- Add 2 µL of IL-2 stock solution (2.1E6 IU/mL) to 198 µL of complete media (i.e., 1:100 dilution), which is RPMI with 1% penicillin-streptomycin and 10% fetal bovine serum (FBS), then proceed to an additional 1:100 dilution by adding 2 µL of IL-2 intermediate solution to 198 µL of complete media to prepare a 7x working solution (210 IU/mL).
- Add 2 µL of CFSE stock solution (5 mM) to 500 µL of plain media (RPMI). Vortex, spin down to remove drops from the cap, then proceed with an additional 1:20 dilution by adding 50 µL of the CFSE intermediate solution to 950 µL of plain RPMI to obtain a 2x working solution of 1 µM. Vortex well and keep protected from light.
2. Isolation of PBMCs as effector cells
NOTE: This assay has been validated for effective use with total PBMCs from healthy controls. However, it is recommended that NK cell content be verified with each PBMC preparation (Figure 1). Also, the volume of whole blood for collection is based on the frequency of NK cells in peripheral whole blood and this may vary from person to person, particularly between healthy donors and patients.
- Collect a minimum of 4 mL of human whole blood in sodium or lithium heparinized blood collection tubes. Store blood samples at room temperature upon collection and use within 30 h of collection.
- Dilute whole blood with 4 mL of PBS (i.e. equal volume of PBS to whole blood).
- Add 4 mL of density gradient solution (see table of materials) to a 15-mL conical tube. Carefully overlay diluted whole blood over the density gradient solution by tilting the tube.
- Centrifuge at 650 x g for 24 min with the brake off.
- Carefully collect the layer of mononuclear cells, which is the thin white layer below the top layer of plasma and platelets, into a new 15-mL conical tube. Bring the volume to 15 mL with PBS.
- Centrifuge at 450 x g for 10 min with the brake on. Aspirate the supernatant and resuspend cells in 0.5 mL of complete media.
- Use an automated cell counter or hemocytometer to count the cells, and adjust the PBMC concentration to 5x106 cells/mL with complete media.
- Check the NK cell content by flow cytometry (see step 4).
- Start the assay within 30 min. Keep cells at room temperature for up to 10 min, or place in a humidified 5% CO2 incubator at 37 °C until ready for use.
3. Isolation of NK cells as effector cells
NOTE: This portion of the protocol is an alternative to using total PBMCs as effector cells. The typical yield from 4 mL of whole blood from a healthy individual is approximately 4x105 NK cells, or approximately 5-15% of PBMCs, though this frequency varies between donors 21. It is recommended that a purity check be performed after the isolation.
- Collect a minimum of 4 mL of human whole blood in sodium or lithium heparinized blood collection tubes. Take 50 µL of whole blood to stain as a pre-enrichment sample.
- Add the enrichment cocktail to blood samples at 50 µL/mL (see table of materials). Mix well and incubate for 10 min at room temperature.
- Add an equal volume of PBS + 2% FBS to dilute the sample and gently mix well.
- Add 4 mL of density gradient solution to a 15-mL conical tube. Carefully overlay the diluted sample on top of the density gradient medium.
- Centrifuge at 1,200 x g for 10 min with the brake off.
- Carefully collect the layer of enriched cells into a new 15-mL conical tube. Wash the cells by bringing the volume up to 50 mL with PBS + 2% FBS.
- Centrifuge at 300 x g for 10 min. Discard the supernatant and repeat the wash.
- Aspirate the supernatant and resuspend cells in 0.5 mL of complete media.
- Using an automated cell counter or hemocytometer, count the cells and adjust the concentration to 5x105 cells/mL. Take 50 µL of purified cell suspension to stain as a post-enrichment sample.
- Keep cells at room temperature for up to 10 min, or place in a humidified 5% CO2 incubator at 37 °C until ready for use.
4. Immunophenotyping for assessment of NK cell content
- Using 50 µL total isolated PBMCs, pre-enrichment whole blood, or purified NK cells, stain with anti-CD56 fluorochrome-conjugated antibody as an NK cell marker. Markers for other cell types and lineages (i.e. CD3+ T cells, CD19+ B cells) may also be included to assess the origin of contamination, if any.
- Incubate for 20 min at 4 °C, and wash by adding 2 mL of PBS + 2% FBS.
- Centrifuge at 450 x g for 5 min.
- For PBMCs or NK cells, aspirate supernatant and resuspend cells in 100 µL of PBS + 2% FBS.
- For whole blood samples, perform red blood cell (RBC) lysis:
- After centrifugation, aspirate the supernatant and resuspend in 2 mL red blood cell lysis buffer (31.75 g of ammonium chloride + 4.16 g of Tris base + 6.65 g of EDTA, dissolved in 5000 mL of deionized water).
- Incubate for 10 min at room temperature.
- Repeat wash twice, aspirate the supernatant, and resuspend lysed sample in 100 µL of PBS + 2% FBS.
NOTE: RBC lysis may also be performed prior to staining. - Proceed with flow cytometric analysis.
5. Thawing, culturing, and harvesting of K562 target cells
- Aliquot 10 mL of complete media into a 15-mL conical tube and allow to warm in a 37 °C water bath. For the thawing and culturing of K562 cells, use only media pre-warmed to 37 °C for all steps.
- Place one vial of frozen K562 cells in the 37 °C water bath until most of it is thawed.
- Transfer the semi-thawed K562 cells to the complete media, pre-warmed to 37 °C, in the 15-mL conical tube. Wash the inside of the cryogenic vial with some pre-warmed media, and add the wash to the 15-mL tube.
- Centrifuge at 140 x g for 7 min with the brake on. Remove the supernatant and resuspend the pellet in 1 mL of complete media.
- Aliquot an appropriate amount of cell suspension to count cells using an automated cell counter or hemocytometer.
- Adjust the concentration to 1x105-2x105 cells/mL and culture in a humidified 5% CO2 incubator at 37 °C for at least 2 days before use. Maintain the culture at this density range for no longer than 3 months, then thaw a new aliquot. Never exceed a cell concentration of 1x106 cells/mL in culture.
- For use in the assay, mix the K562 cell culture well with a 10-mL serological pipette and remove an aliquot to count and assess cell viability using an automated cell counter or hemocytometer and Trypan Blue staining.
- If the viability is less than 85%, proceed with the following dead cell removal steps.
- Add 15 mL of density gradient solution to 50-mL conical tubes, as needed. Collect all cells from culture and slowly overlay the cell suspension over the density gradient solution by tilting the tube.
- Centrifuge at 650 x gfor 24 min with brake off. Carefully collect the layer of live cells into a new 15-mL conical tube. Bring the volume to 50 mL with PBS.
- Centrifuge at 450 x g for 10 min with the brake on. Remove the supernatant and resuspend the pellet in 1 mL of complete media. Count the cells and check cell viability (as in 5.7).
- Culture in a humidified 5% CO2 incubator at 37 °C for at least 2 days before use.
- If the viability is less than 85%, proceed with the following dead cell removal steps.
- Collect 5x105 K562 target cells and centrifuge at 140 x g for 7 min. Aspirate the supernatant and resuspend the cell pellet in 500 µL in media with 1% FBS.
6. Labeling of K562 target cells
NOTE: Make sure the K562 cells are well resuspended before adding the CFSE working solution.
- Pipette 500 µL of the CFSE working solution to the 500 µL of K562 cells for a final concentration of 0.5 µM. Using the same 1000-1250 µL tip, mix immediately by gently pipetting up and down 3-5 times.
- Incubate in the 37 °C water bath or in a humidified 5% CO2 incubator at 37 °C for 10 min in the dark.
NOTE: Since the temperature equilibration lag time is longer in the incubator compared to the water bath, incubation time might need to be adjusted especially for volumes > 1 mL. - Quench the labeling reaction with 10 mL of complete media for 10 min in the dark at room temperature.
- Centrifuge the labeled K562 cells at 140 x g for 7 min. Remove the supernatant and resuspend in 1 mL of complete media.
- Count the cells using an automated cell counter or hemocytometer and bring the concentration to 1x105 cells/mL in complete media.
- Place cells in a humidified 5% CO2 incubator at 37 °C until ready for use. Cells can be kept at room temperature for up to 10 min, or in in a humidified CO2 incubator at 37 °C until ready for use. Do not refrigerate.
7. Plating
NOTE: If NK cells are limiting, the concentration of both K562 and NK cells can be halved, so that half of the cells are plated in the assay. The assay yields comparable results with half the amount of cells (as long as the ratios are kept consistent).
- If using total PBMCs, label a 96-well, U-bottom, tissue culture-treated plate for the following conditions per well: (1) Effector (E) : Target (T) = 50 : 1 + IL-2 - positive control for NK cell cytotoxicity; (2) E : T = 50 : 1; (3) E : T = 25 : 1; (4) E : T = 12.5 : 1; (5) E : T = 6.25 : 1; (6) T - target cells only - negative control for K562 death; (7) E - effector cells only; (8) T + Tween - Positive control for K562 death
- If using purified NK cells, label a 96-well, U-bottom, tissue culture treated plate for the following conditions per well: (1) Effector (E) : Target (T) = 5: 1 + IL-2 - positive control for NK cell cytotoxicity; (2) E : T = 5 : 1; (3) E : T = 2.5 : 1; (4) E : T = 1.25 : 1; (5) E : T = 0.625: 1; (6) T - target cells only - negative control for K562 death; (7) E - effector cells only; (8) T + Tween - positive control for K562 death
- Add 100 µL of complete media to conditions 3, 4, 5, 6, 7. For this and all subsequent steps, (recommended) use a multichannel pipette.
- Add 100 µL of effector cells (PBMCs at 5x106 cells/mL or purified NK cells at 5x105 cells/mL) to conditions 1, 2, 3. Add effector cells for conditions 4-7 by serial dilution. Do not change tips from condition to condition.
- Mix condition 3, take 100 µL, and transfer to condition 4.
- Mix condition 4, take 100 µL, and transfer to condition 5.
- Mix condition 5, take 100 µL, and transfer to condition 7; Condition 6 does not have effector cells.
- Add 100 µL of prepared 2x 0.2% Tween working solution to condition 8 (final concentration: 0.1% Tween-20); condition 8 does not have effector cells.
- Add 30 µL of IL-2 working solution to condition 1. IL-2 should be added to the effector cell suspension prior to the addition of target cell suspension (final concentration: 27 IU/mL).
- Add 100 µL of target cells (labeled K562 cells at 1x105 cells/mL) to conditions 1, 2, 3, 4, 5, 6, 8. Condition 7 does not have target cells.
- Mix all wells with a multichannel pipette and centrifuge the plate at 120 x g for 2 min.
- Incubate in a humidified CO2 incubator at 37 °C for 4 h. At the end of the incubation, place the plate on ice and proceed to viability staining within 30 min.
8. Staining for viability and acquisition
- Prepare dead cell stain (see table of materials) by adding 3 µL of the 5 µM stock solution to 600 µL of PBS.
- Add 50 µL of diluted dead cell stain to each well for a final volume of 250 µL and a final concentration of 0.005 µM. Mix well with a multichannel pipette.
- Proceed with flow cytometric analysis. Using a flow cytometer, collect at least 1,000 events in the target cell gate (Figure 2) for statistically meaningful results.
Access restricted. Please log in or start a trial to view this content.
Resultados
Antes de establecer el ensayo, se recomienda evaluar contenido de células NK en la población de efector de elección. La figura 1 muestra una típica coloración CD56 antes (azul claro) y después de enriquecimiento de células NK (rojo). Las células NK constituyen hasta un 15% de PBMCs y deben ser por lo menos 80% de pureza después de enriquecimiento.
El análisis cytometric del flujo en este e...
Access restricted. Please log in or start a trial to view this content.
Discusión
El método descrito aquí proporciona una alternativa sencilla y rentable para el ensayo de lanzamiento tradicionales 51Cr para evaluar la actividad citotóxica de células NK. Este método es sensible, reproducible y menos desperdiciador de tiempo que los anteriores métodos estándar, como CRA y puede ser utilizado para ambas clínicas y aplicaciones de investigación.
Mientras que el ensayo trabaja con total PBMCs y enriquecida de las células NK, la opción de usar PBMCs sin la ...
Access restricted. Please log in or start a trial to view this content.
Divulgaciones
Los autores no declaran conflictos de interés financiero.
Agradecimientos
Nos gustaría agradecer a Jill Narciso, centro de inmunogenética de UCLA, por su asistencia con la preparación del manuscrito.
Access restricted. Please log in or start a trial to view this content.
Materiales
| Name | Company | Catalog Number | Comments |
| Phosphate-buffered Saline (1x, w/o Ca2+ and Mg2+) | Corning (Cellgro) | 21-040-CM | |
| Ficoll-Paque PLUS | GE Healthcare | 17-1440-02 | |
| Tween-20 | Sigma | BP337-100 | |
| RPMI 1640 Media | Corning (Cellgro) | 10-040-CV | |
| Heat-inactivated Fetal Bovine Serum | Omega Scientific | FB-02 | |
| Penicillin Streptomycin | Life Technologies | 15140-163 | Stock solution at 10,000 U/mL |
| IL-2 | R&D Systems | 202-IL-050 | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA with BSA as a carrier protein. Reconstitute with 500 ul at 100 μg/mL in sterile 100 mM Acetic Acid containing at least 0.1% bovine serum albumin (2.1x10E6 IU/ml) |
| K562 Cells | ATCC | CCL-243 | Cancer cell line |
| T-75 cell culture flasks | Corning | 431464 | |
| CFSE cell proliferation kit | Life Technologies (CellTrace) | C34554 | Reconstitute I vial with 18 ul DMSO to prepare a 5mM stock solution. Do not freeze/thaw. |
| Sytox Red | Life Technologies | S34859 | Stock solution is provided at 5 μM in 1 mL DMSO. The DMSO solution may be subjected to multiple freeze-thaw cycles without reagent degradation. |
| Sodium/lithium heparin blood collection tubes | BD | 02-687-95 | |
| U-bottom 96-well plate | Corning | CLS3897 | |
| Serological pipettes | BD Falcon | ||
| Polystyrene round-bottom tubes (5mL) | BD Falcon | 14959-5 | |
| 50 mL polypropylene conical tube | BD Falcon | 352070 | |
| 15 mL polypropylene conical tube | BD Falcon | 352097 | |
| Reagent reservoir | USA Scientific | 2321-2230 | |
| Human NK cell enrichment cocktail | StemCell Technologies (RosetteSep) | 15065 |
Referencias
- Iannello, A., Debbeche, O., Samarani, S., Ahmad, A. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J Leukoc Biol. 84 (1), 1-26 (2008).
- Caligiuri, M. A. Human natural killer cells. Blood. 112 (3), 461-469 (2008).
- Topham, N. J., Hewitt, E. W. Natural killer cell cytotoxicity: how do they pull the trigger? Immunology. 128 (1), 7-15 (2009).
- Warren, H. S., Smyth, M. J. NK cells and apoptosis. Immunol Cell Biol. 77 (1), 64-75 (1999).
- Tognarelli, S., Jacobs, B., Staiger, N., Ullrich, E. Flow Cytometry-based Assay for the Monitoring of NK Cell Functions. J Vis Exp. (116), (2016).
- Somanchi, S. S., McCulley, K. J., Somanchi, A., Chan, L. L., Lee, D. A. A Novel Method for Assessment of Natural Killer Cell Cytotoxicity Using Image Cytometry. PLoS One. 10 (10), e0141074(2015).
- Alter, G., Malenfant, J. M., Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 294 (1-2), 15-22 (2004).
- Atkinson, E. A., Gerrard, J. M., Hildes, G. E., Greenberg, A. H. Studies of the mechanism of natural killer (NK) degranulation and cytotoxicity. J Leukoc Biol. 47 (1), 39-48 (1990).
- Kim, G. G., Donnenberg, V. S., Donnenberg, A. D., Gooding, W., Whiteside, T. L. A novel multiparametric flow cytometry-based cytotoxicity assay simultaneously immunophenotypes effector cells: comparisons to a 4 h 51Cr-release assay. J Immunol Methods. 325 (1-2), 51-66 (2007).
- Kane, K. L., Ashton, F. A., Schmitz, J. L., Folds, J. D. Determination of natural killer cell function by flow cytometry. Clin Diagn Lab Immunol. 3 (3), 295-300 (1996).
- Jang, Y. Y., et al. An improved flow cytometry-based natural killer cytotoxicity assay involving calcein AM staining of effector cells. Ann Clin Lab Sci. 42 (1), 42-49 (2012).
- Korzeniewski, C., Callewaert, D. M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 64 (3), 313-320 (1983).
- Karimi, M. A., et al. Measuring cytotoxicity by bioluminescence imaging outperforms the standard chromium-51 release assay. PLoS One. 9 (2), e89357(2014).
- Oppenheim, D. E., et al. Glyco-engineered anti-EGFR mAb elicits ADCC by NK cells from colorectal cancer patients irrespective of chemotherapy. Br J Cancer. 110 (5), 1221-1227 (2014).
- West, W. H., Cannon, G. B., Kay, H. D., Bonnard, G. D., Herberman, R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 118 (1), 355-361 (1977).
- Jedema, I., van der Werff, N. M., Barge, R. M., Willemze, R., Falkenburg, J. H. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood. 103 (7), 2677-2682 (2004).
- Lecoeur, H., Fevrier, M., Garcia, S., Riviere, Y., Gougeon, M. L. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J Immunol Methods. 253 (1-2), 177-187 (2001).
- Carotta, S. Targeting NK Cells for Anticancer Immunotherapy: Clinical and Preclinical Approaches. Front Immunol. 7, 152(2016).
- Mandal, A., Viswanathan, C. Natural killer cells: In health and disease. Hematol Oncol Stem Cell Ther. 8 (2), 47-55 (2015).
- Rezvani, K., Rouce, R. H. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front Immunol. 6, 578(2015).
- Angelo, L. S., et al. Practical NK cell phenotyping and variability in healthy adults. Immunol Res. 62 (3), 341-356 (2015).
- Zons, P., et al. Comparison of europium and chromium release assays: cytotoxicity in healthy individuals and patients with cervical carcinoma. Clin Diagn Lab Immunol. 4 (2), 202-207 (1997).
- Yovel, G., Shakhar, K., Ben-Eliyahu, S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. 81 (2), 254-262 (2001).
- Laue, T., et al. Altered NK cell function in obese healthy humans. BMC Obes. 2, 1(2015).
- Hazeldine, J., Lord, J. M. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res Rev. 12 (4), 1069-1078 (2013).
Access restricted. Please log in or start a trial to view this content.
Erratum
Formal Correction: Erratum: A Flow Cytometry-Based Cytotoxicity Assay for the Assessment of Human NK Cell Activity
Posted by JoVE Editors on 9/10/2017. Citeable Link.
An erratum was issued for: A Flow Cytometry-Based Cytotoxicity Assay for the Assessment of Human NK Cell Activity. Figure 4 has been corrected to show background-corrected data.
Figure 4 was updated from:
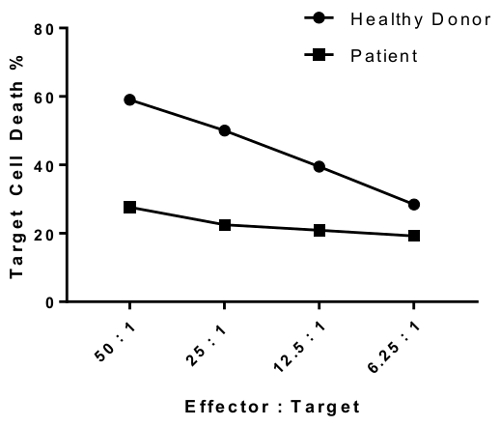
to:
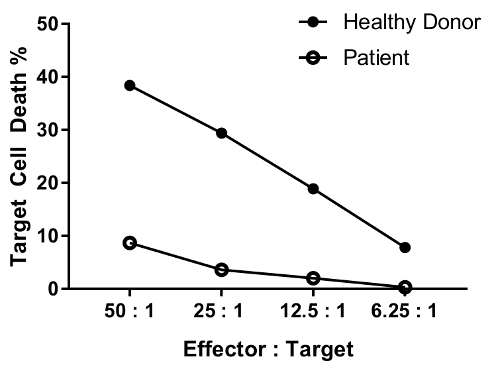
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoThis article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados