È necessario avere un abbonamento a JoVE per visualizzare questo. Accedi o inizia la tua prova gratuita.
Method Article
Un'analisi di citotossicità di base di citometria a flusso per la valutazione dell'attività delle cellule NK umane
In questo articolo
Erratum Notice
Riepilogo
Un metodo di basato su citometria di flusso per determinare quantitativamente l'attività citotossica delle cellule natural killer di umane è mostrato qui.
Abstract
All'interno del sistema immunitario innato, linfociti effettori conosciuti come cellule natural killer (NK) svolgono un ruolo essenziale nella difesa dell'ospite contro le cellule aberranti, in particolare eliminando tumorali e cellule infette viralmente. Circa 30 difetti noti monogenici, insieme a una serie di altre condizioni patologiche, causano la mancanza delle cellule NK sia funzionale o classica, che si manifesta ridotta o assente attività citotossica. Storicamente, la citotossicità è stata studiata con metodi radioattivi, che sono ingombranti, costosi e potenzialmente pericolose. Questo articolo viene descritto un metodo di base di cytometry di flusso aerodinamico, clinicamente applicabile per quantificare l'attività citotossica delle cellule NK. In questo saggio, cellule mononucleari del sangue periferico (PBMCs) o preparazioni purificate di cellule NK sono co-incubate a diversi rapporti con una linea cellulare di tumore destinazione conosciuta ad essere sensibili alla citotossicità cellulo-mediata NK (NKCC). Le cellule bersaglio sono pre-etichettate con un colorante fluorescente per permettere la loro distinzione dalle cellule effettrici (cellule NK). Dopo il periodo di incubazione, le cellule bersaglio uccisi sono identificate da una macchia di acido nucleico, che permea in modo specifico le cellule morte. Questo metodo è favorevole alla sia diagnostico e applicazioni di ricerca e, grazie alle funzionalità multi-parametro di citometria a flusso, presenta il vantaggio aggiunto di potenzialmente consentire un'analisi più approfondita del fenotipo delle cellule NK e funzione.
Introduzione
Cellule natural killer (NK) sono un sottoinsieme sofisticato dei linfociti umani innati criticamente coinvolti nell'eliminazione delle cellule infettate viralmente, cellule trasformate e altri patogeni minacce 1,2. Granuli litici delle cellule di NK casa proteine citotossiche, come perforine e granzimi. Al momento dell'attivazione, le cellule NK formano una complessa interazione con i loro obiettivi conosciuto come sinapsi immunologica, per cui queste molecole citolitiche localmente vengono rilasciate, con conseguente lisi delle cellule bersaglio diretto e apoptosi, insieme al rilascio di citochine e chemochine e, infine, l'induzione di un infiammatorio stato 1,3,4.
Attivazione delle cellule NK coinvolge una stringa complessa di attivazione e inibitorie interazioni tra NK delle cellule recettori e ligandi espressi sulla superficie delle cellule bersaglio, formando un sistema strettamente regolato. Uno dei più studiati meccanismi di attivazione delle cellule NK è il "sé mancante". Infatti, mancanza di rilevazione di classe che maggiore istocompatibilità (MHC), o umano del leucocita (HLA) l'antigene molecole, il infetti o trasformato la citotossicità delle cellule NK di cellule trigger. Tumore e cellule infettate da virus generalmente downregulate questi antigeni di fuggire immunità comunicata per cellule di T, diventando così NK primario delle cellule target 1,3,4.
Valutazione della funzione delle cellule NK è principalmente suddivise in degranulazione o citotossicità saggi. Tuttavia, test di degranulazione, quali il rilevamento di cytometric di flusso del marcatore associato degranulazione CD107a, sono indicativi dell'attivazione delle cellule NK e non della loro funzione finale, l'uccisione diretta di destinazione celle 5,6,7,8. Quindi, questa limitazione ha attirato gli investigatori a saggi di citotossicità come un'alternativa più eloquente e più diretto.
Da sempre "gold standard" per la valutazione della attività citotossica cellulo-mediata delle cellule NK e T è il test di rilascio di cromo (CRA). CRA coinvolge radioattivo etichettatura delle cellule bersaglio con 51Cr e co-incubarle con cellule effettrici. Questo test è immersa nel principio che la lisi delle cellule provoca il rilascio di legato alle proteine 51Cr nel surnatante, che può essere misurato contando gamma. Questo test, mentre efficaci, è problematico per una serie di motivi: costi elevati di materiale, manipolazione e smaltimento di radioattivo 51Cr, rilascio spontaneo di 51Cr e difficile standardizzazione - rendendolo del tutto impraticabile 9,10.
Una serie di saggi non radioattivo, che coinvolgono etichettatura fluorescente, rilascio di enzimi e anche bioluminescenza, allora è stata sviluppata come alternative al CRA 11,12,13,14. Descriviamo qui un metodo di basato su citometria di flusso per la misura di attività delle cellule NK citotossica sulle cellule bersaglio K562 che è semplice, sensibile e riproducibile. Le cellule K562 sono una cellula umana eritroleucemiche linea con riduzione dell'espressione di molecole HLA di classe I ed espressione intensificata di ligandi per recettori NK attivatori, che li rende particolarmente suscettibili a NK Citotossicità cellulo-mediata 15. In questa analisi, le cellule K562 sono pre-etichettate con carboxyfluorescein diacetate succinimidyl ester (CFSE) e co-coltivate a vari rapporti con entrambi cellule mononucleari del sangue periferico (PBMCs) o purificata di cellule NK 1. CFSE è un colorante fluorescente stabile, legame che consente di discriminazione delle cellule bersaglio da effettore NK cellule 16,17. Dopo la co-incubazione, una macchia di acido nucleico, specificamente che permea la membrana di cellule morte, viene utilizzata per identificare le celle di destinazione ucciso (Vedi tabella dei materiali). I campioni vengono quindi acquisiti su un citometro a flusso per determinare la percentuale di morti (cioè, macchia +) CFSE + cellule bersaglio.
Questa analisi può essere usata come uno screening diagnostico per malattie monogenici difetti che interessano il compartimento cellulare NK, di cui ci sono circa 30 difetti noti causando carenza di cellule NK o funzionale o classica, e per il lymphohistiocytosis hemophagocytic primario o secondario. È anche utile indagare l'attività delle cellule NK in pazienti con infezioni virali da herpes ricorrente, grave, di valutare la ricostituzione immunitaria dopo trapianto di cellule ematopoietiche o post immunomodulatori terapia 18,19,20e per una miriade di applicazioni di ricerca di base.
Access restricted. Please log in or start a trial to view this content.
Protocollo
Samples were collected according to the ethical guidelines established by the UCLA Human Research Protection Program and IRB approved.
1. Preparation of reagents
NOTE: Unless otherwise stated, all reagents should be allowed to equilibrate at room temperature prior to use. All reagents must remain sterile.
- Prepare a 2x working solution of Tween-20 (i.e., 0.2%) by adding 10 µL of Tween-20 solution to 5 mL of phosphate-buffered saline (PBS) without calcium and magnesium with a p20 pipette.
- Given the high viscosity of Tween-20, take the following steps to ensure accuracy: collect slowly to avoid bubble formation, do not submerge the entire tip to avoid carry-over, and pipette up and down several times in PBS to wash out all Tween-20 from the tip.
- Add 2 µL of IL-2 stock solution (2.1E6 IU/mL) to 198 µL of complete media (i.e., 1:100 dilution), which is RPMI with 1% penicillin-streptomycin and 10% fetal bovine serum (FBS), then proceed to an additional 1:100 dilution by adding 2 µL of IL-2 intermediate solution to 198 µL of complete media to prepare a 7x working solution (210 IU/mL).
- Add 2 µL of CFSE stock solution (5 mM) to 500 µL of plain media (RPMI). Vortex, spin down to remove drops from the cap, then proceed with an additional 1:20 dilution by adding 50 µL of the CFSE intermediate solution to 950 µL of plain RPMI to obtain a 2x working solution of 1 µM. Vortex well and keep protected from light.
2. Isolation of PBMCs as effector cells
NOTE: This assay has been validated for effective use with total PBMCs from healthy controls. However, it is recommended that NK cell content be verified with each PBMC preparation (Figure 1). Also, the volume of whole blood for collection is based on the frequency of NK cells in peripheral whole blood and this may vary from person to person, particularly between healthy donors and patients.
- Collect a minimum of 4 mL of human whole blood in sodium or lithium heparinized blood collection tubes. Store blood samples at room temperature upon collection and use within 30 h of collection.
- Dilute whole blood with 4 mL of PBS (i.e. equal volume of PBS to whole blood).
- Add 4 mL of density gradient solution (see table of materials) to a 15-mL conical tube. Carefully overlay diluted whole blood over the density gradient solution by tilting the tube.
- Centrifuge at 650 x g for 24 min with the brake off.
- Carefully collect the layer of mononuclear cells, which is the thin white layer below the top layer of plasma and platelets, into a new 15-mL conical tube. Bring the volume to 15 mL with PBS.
- Centrifuge at 450 x g for 10 min with the brake on. Aspirate the supernatant and resuspend cells in 0.5 mL of complete media.
- Use an automated cell counter or hemocytometer to count the cells, and adjust the PBMC concentration to 5x106 cells/mL with complete media.
- Check the NK cell content by flow cytometry (see step 4).
- Start the assay within 30 min. Keep cells at room temperature for up to 10 min, or place in a humidified 5% CO2 incubator at 37 °C until ready for use.
3. Isolation of NK cells as effector cells
NOTE: This portion of the protocol is an alternative to using total PBMCs as effector cells. The typical yield from 4 mL of whole blood from a healthy individual is approximately 4x105 NK cells, or approximately 5-15% of PBMCs, though this frequency varies between donors 21. It is recommended that a purity check be performed after the isolation.
- Collect a minimum of 4 mL of human whole blood in sodium or lithium heparinized blood collection tubes. Take 50 µL of whole blood to stain as a pre-enrichment sample.
- Add the enrichment cocktail to blood samples at 50 µL/mL (see table of materials). Mix well and incubate for 10 min at room temperature.
- Add an equal volume of PBS + 2% FBS to dilute the sample and gently mix well.
- Add 4 mL of density gradient solution to a 15-mL conical tube. Carefully overlay the diluted sample on top of the density gradient medium.
- Centrifuge at 1,200 x g for 10 min with the brake off.
- Carefully collect the layer of enriched cells into a new 15-mL conical tube. Wash the cells by bringing the volume up to 50 mL with PBS + 2% FBS.
- Centrifuge at 300 x g for 10 min. Discard the supernatant and repeat the wash.
- Aspirate the supernatant and resuspend cells in 0.5 mL of complete media.
- Using an automated cell counter or hemocytometer, count the cells and adjust the concentration to 5x105 cells/mL. Take 50 µL of purified cell suspension to stain as a post-enrichment sample.
- Keep cells at room temperature for up to 10 min, or place in a humidified 5% CO2 incubator at 37 °C until ready for use.
4. Immunophenotyping for assessment of NK cell content
- Using 50 µL total isolated PBMCs, pre-enrichment whole blood, or purified NK cells, stain with anti-CD56 fluorochrome-conjugated antibody as an NK cell marker. Markers for other cell types and lineages (i.e. CD3+ T cells, CD19+ B cells) may also be included to assess the origin of contamination, if any.
- Incubate for 20 min at 4 °C, and wash by adding 2 mL of PBS + 2% FBS.
- Centrifuge at 450 x g for 5 min.
- For PBMCs or NK cells, aspirate supernatant and resuspend cells in 100 µL of PBS + 2% FBS.
- For whole blood samples, perform red blood cell (RBC) lysis:
- After centrifugation, aspirate the supernatant and resuspend in 2 mL red blood cell lysis buffer (31.75 g of ammonium chloride + 4.16 g of Tris base + 6.65 g of EDTA, dissolved in 5000 mL of deionized water).
- Incubate for 10 min at room temperature.
- Repeat wash twice, aspirate the supernatant, and resuspend lysed sample in 100 µL of PBS + 2% FBS.
NOTE: RBC lysis may also be performed prior to staining. - Proceed with flow cytometric analysis.
5. Thawing, culturing, and harvesting of K562 target cells
- Aliquot 10 mL of complete media into a 15-mL conical tube and allow to warm in a 37 °C water bath. For the thawing and culturing of K562 cells, use only media pre-warmed to 37 °C for all steps.
- Place one vial of frozen K562 cells in the 37 °C water bath until most of it is thawed.
- Transfer the semi-thawed K562 cells to the complete media, pre-warmed to 37 °C, in the 15-mL conical tube. Wash the inside of the cryogenic vial with some pre-warmed media, and add the wash to the 15-mL tube.
- Centrifuge at 140 x g for 7 min with the brake on. Remove the supernatant and resuspend the pellet in 1 mL of complete media.
- Aliquot an appropriate amount of cell suspension to count cells using an automated cell counter or hemocytometer.
- Adjust the concentration to 1x105-2x105 cells/mL and culture in a humidified 5% CO2 incubator at 37 °C for at least 2 days before use. Maintain the culture at this density range for no longer than 3 months, then thaw a new aliquot. Never exceed a cell concentration of 1x106 cells/mL in culture.
- For use in the assay, mix the K562 cell culture well with a 10-mL serological pipette and remove an aliquot to count and assess cell viability using an automated cell counter or hemocytometer and Trypan Blue staining.
- If the viability is less than 85%, proceed with the following dead cell removal steps.
- Add 15 mL of density gradient solution to 50-mL conical tubes, as needed. Collect all cells from culture and slowly overlay the cell suspension over the density gradient solution by tilting the tube.
- Centrifuge at 650 x gfor 24 min with brake off. Carefully collect the layer of live cells into a new 15-mL conical tube. Bring the volume to 50 mL with PBS.
- Centrifuge at 450 x g for 10 min with the brake on. Remove the supernatant and resuspend the pellet in 1 mL of complete media. Count the cells and check cell viability (as in 5.7).
- Culture in a humidified 5% CO2 incubator at 37 °C for at least 2 days before use.
- If the viability is less than 85%, proceed with the following dead cell removal steps.
- Collect 5x105 K562 target cells and centrifuge at 140 x g for 7 min. Aspirate the supernatant and resuspend the cell pellet in 500 µL in media with 1% FBS.
6. Labeling of K562 target cells
NOTE: Make sure the K562 cells are well resuspended before adding the CFSE working solution.
- Pipette 500 µL of the CFSE working solution to the 500 µL of K562 cells for a final concentration of 0.5 µM. Using the same 1000-1250 µL tip, mix immediately by gently pipetting up and down 3-5 times.
- Incubate in the 37 °C water bath or in a humidified 5% CO2 incubator at 37 °C for 10 min in the dark.
NOTE: Since the temperature equilibration lag time is longer in the incubator compared to the water bath, incubation time might need to be adjusted especially for volumes > 1 mL. - Quench the labeling reaction with 10 mL of complete media for 10 min in the dark at room temperature.
- Centrifuge the labeled K562 cells at 140 x g for 7 min. Remove the supernatant and resuspend in 1 mL of complete media.
- Count the cells using an automated cell counter or hemocytometer and bring the concentration to 1x105 cells/mL in complete media.
- Place cells in a humidified 5% CO2 incubator at 37 °C until ready for use. Cells can be kept at room temperature for up to 10 min, or in in a humidified CO2 incubator at 37 °C until ready for use. Do not refrigerate.
7. Plating
NOTE: If NK cells are limiting, the concentration of both K562 and NK cells can be halved, so that half of the cells are plated in the assay. The assay yields comparable results with half the amount of cells (as long as the ratios are kept consistent).
- If using total PBMCs, label a 96-well, U-bottom, tissue culture-treated plate for the following conditions per well: (1) Effector (E) : Target (T) = 50 : 1 + IL-2 - positive control for NK cell cytotoxicity; (2) E : T = 50 : 1; (3) E : T = 25 : 1; (4) E : T = 12.5 : 1; (5) E : T = 6.25 : 1; (6) T - target cells only - negative control for K562 death; (7) E - effector cells only; (8) T + Tween - Positive control for K562 death
- If using purified NK cells, label a 96-well, U-bottom, tissue culture treated plate for the following conditions per well: (1) Effector (E) : Target (T) = 5: 1 + IL-2 - positive control for NK cell cytotoxicity; (2) E : T = 5 : 1; (3) E : T = 2.5 : 1; (4) E : T = 1.25 : 1; (5) E : T = 0.625: 1; (6) T - target cells only - negative control for K562 death; (7) E - effector cells only; (8) T + Tween - positive control for K562 death
- Add 100 µL of complete media to conditions 3, 4, 5, 6, 7. For this and all subsequent steps, (recommended) use a multichannel pipette.
- Add 100 µL of effector cells (PBMCs at 5x106 cells/mL or purified NK cells at 5x105 cells/mL) to conditions 1, 2, 3. Add effector cells for conditions 4-7 by serial dilution. Do not change tips from condition to condition.
- Mix condition 3, take 100 µL, and transfer to condition 4.
- Mix condition 4, take 100 µL, and transfer to condition 5.
- Mix condition 5, take 100 µL, and transfer to condition 7; Condition 6 does not have effector cells.
- Add 100 µL of prepared 2x 0.2% Tween working solution to condition 8 (final concentration: 0.1% Tween-20); condition 8 does not have effector cells.
- Add 30 µL of IL-2 working solution to condition 1. IL-2 should be added to the effector cell suspension prior to the addition of target cell suspension (final concentration: 27 IU/mL).
- Add 100 µL of target cells (labeled K562 cells at 1x105 cells/mL) to conditions 1, 2, 3, 4, 5, 6, 8. Condition 7 does not have target cells.
- Mix all wells with a multichannel pipette and centrifuge the plate at 120 x g for 2 min.
- Incubate in a humidified CO2 incubator at 37 °C for 4 h. At the end of the incubation, place the plate on ice and proceed to viability staining within 30 min.
8. Staining for viability and acquisition
- Prepare dead cell stain (see table of materials) by adding 3 µL of the 5 µM stock solution to 600 µL of PBS.
- Add 50 µL of diluted dead cell stain to each well for a final volume of 250 µL and a final concentration of 0.005 µM. Mix well with a multichannel pipette.
- Proceed with flow cytometric analysis. Using a flow cytometer, collect at least 1,000 events in the target cell gate (Figure 2) for statistically meaningful results.
Access restricted. Please log in or start a trial to view this content.
Risultati
Prima di impostare il dosaggio, si consiglia vivamente che contenuto delle cellule NK essere valutata nella popolazione dell'effettore di scelta. La figura 1 Mostra una tipica colorazione CD56 prima (luce blu) e dopo arricchimento di cellule NK (rosso). Le cellule NK comprendono fino a 15% di PBMCs e devono essere almeno 80% puro dopo arricchimento.
Analisi cytometric di flusso in questo test compor...
Access restricted. Please log in or start a trial to view this content.
Discussione
Il metodo qui descritto fornisce un'alternativa semplice e conveniente per il test di rilascio di Cr tradizionale 51per valutare l'attività citotossica delle cellule NK. Questo metodo è sensibile, riproducibile e richiede meno tempo rispetto ai metodi standard precedenti, come CRA e può essere usato per entrambi clinici e applicazioni di ricerca.
Mentre il dosaggio funziona sia con totale PBMCs e arricchito le cellule NK, l'opzione per utilizzare PBMCs senza la necessità di puri...
Access restricted. Please log in or start a trial to view this content.
Divulgazioni
Gli autori non dichiarano conflitti di interesse finanziario.
Riconoscimenti
Vorremmo ringraziare Jill Narciso, UCLA Immunogenetics Center, per la sua assistenza con la preparazione del manoscritto.
Access restricted. Please log in or start a trial to view this content.
Materiali
| Name | Company | Catalog Number | Comments |
| Phosphate-buffered Saline (1x, w/o Ca2+ and Mg2+) | Corning (Cellgro) | 21-040-CM | |
| Ficoll-Paque PLUS | GE Healthcare | 17-1440-02 | |
| Tween-20 | Sigma | BP337-100 | |
| RPMI 1640 Media | Corning (Cellgro) | 10-040-CV | |
| Heat-inactivated Fetal Bovine Serum | Omega Scientific | FB-02 | |
| Penicillin Streptomycin | Life Technologies | 15140-163 | Stock solution at 10,000 U/mL |
| IL-2 | R&D Systems | 202-IL-050 | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA with BSA as a carrier protein. Reconstitute with 500 ul at 100 μg/mL in sterile 100 mM Acetic Acid containing at least 0.1% bovine serum albumin (2.1x10E6 IU/ml) |
| K562 Cells | ATCC | CCL-243 | Cancer cell line |
| T-75 cell culture flasks | Corning | 431464 | |
| CFSE cell proliferation kit | Life Technologies (CellTrace) | C34554 | Reconstitute I vial with 18 ul DMSO to prepare a 5mM stock solution. Do not freeze/thaw. |
| Sytox Red | Life Technologies | S34859 | Stock solution is provided at 5 μM in 1 mL DMSO. The DMSO solution may be subjected to multiple freeze-thaw cycles without reagent degradation. |
| Sodium/lithium heparin blood collection tubes | BD | 02-687-95 | |
| U-bottom 96-well plate | Corning | CLS3897 | |
| Serological pipettes | BD Falcon | ||
| Polystyrene round-bottom tubes (5mL) | BD Falcon | 14959-5 | |
| 50 mL polypropylene conical tube | BD Falcon | 352070 | |
| 15 mL polypropylene conical tube | BD Falcon | 352097 | |
| Reagent reservoir | USA Scientific | 2321-2230 | |
| Human NK cell enrichment cocktail | StemCell Technologies (RosetteSep) | 15065 |
Riferimenti
- Iannello, A., Debbeche, O., Samarani, S., Ahmad, A. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J Leukoc Biol. 84 (1), 1-26 (2008).
- Caligiuri, M. A. Human natural killer cells. Blood. 112 (3), 461-469 (2008).
- Topham, N. J., Hewitt, E. W. Natural killer cell cytotoxicity: how do they pull the trigger? Immunology. 128 (1), 7-15 (2009).
- Warren, H. S., Smyth, M. J. NK cells and apoptosis. Immunol Cell Biol. 77 (1), 64-75 (1999).
- Tognarelli, S., Jacobs, B., Staiger, N., Ullrich, E. Flow Cytometry-based Assay for the Monitoring of NK Cell Functions. J Vis Exp. (116), (2016).
- Somanchi, S. S., McCulley, K. J., Somanchi, A., Chan, L. L., Lee, D. A. A Novel Method for Assessment of Natural Killer Cell Cytotoxicity Using Image Cytometry. PLoS One. 10 (10), e0141074(2015).
- Alter, G., Malenfant, J. M., Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 294 (1-2), 15-22 (2004).
- Atkinson, E. A., Gerrard, J. M., Hildes, G. E., Greenberg, A. H. Studies of the mechanism of natural killer (NK) degranulation and cytotoxicity. J Leukoc Biol. 47 (1), 39-48 (1990).
- Kim, G. G., Donnenberg, V. S., Donnenberg, A. D., Gooding, W., Whiteside, T. L. A novel multiparametric flow cytometry-based cytotoxicity assay simultaneously immunophenotypes effector cells: comparisons to a 4 h 51Cr-release assay. J Immunol Methods. 325 (1-2), 51-66 (2007).
- Kane, K. L., Ashton, F. A., Schmitz, J. L., Folds, J. D. Determination of natural killer cell function by flow cytometry. Clin Diagn Lab Immunol. 3 (3), 295-300 (1996).
- Jang, Y. Y., et al. An improved flow cytometry-based natural killer cytotoxicity assay involving calcein AM staining of effector cells. Ann Clin Lab Sci. 42 (1), 42-49 (2012).
- Korzeniewski, C., Callewaert, D. M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 64 (3), 313-320 (1983).
- Karimi, M. A., et al. Measuring cytotoxicity by bioluminescence imaging outperforms the standard chromium-51 release assay. PLoS One. 9 (2), e89357(2014).
- Oppenheim, D. E., et al. Glyco-engineered anti-EGFR mAb elicits ADCC by NK cells from colorectal cancer patients irrespective of chemotherapy. Br J Cancer. 110 (5), 1221-1227 (2014).
- West, W. H., Cannon, G. B., Kay, H. D., Bonnard, G. D., Herberman, R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 118 (1), 355-361 (1977).
- Jedema, I., van der Werff, N. M., Barge, R. M., Willemze, R., Falkenburg, J. H. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood. 103 (7), 2677-2682 (2004).
- Lecoeur, H., Fevrier, M., Garcia, S., Riviere, Y., Gougeon, M. L. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J Immunol Methods. 253 (1-2), 177-187 (2001).
- Carotta, S. Targeting NK Cells for Anticancer Immunotherapy: Clinical and Preclinical Approaches. Front Immunol. 7, 152(2016).
- Mandal, A., Viswanathan, C. Natural killer cells: In health and disease. Hematol Oncol Stem Cell Ther. 8 (2), 47-55 (2015).
- Rezvani, K., Rouce, R. H. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front Immunol. 6, 578(2015).
- Angelo, L. S., et al. Practical NK cell phenotyping and variability in healthy adults. Immunol Res. 62 (3), 341-356 (2015).
- Zons, P., et al. Comparison of europium and chromium release assays: cytotoxicity in healthy individuals and patients with cervical carcinoma. Clin Diagn Lab Immunol. 4 (2), 202-207 (1997).
- Yovel, G., Shakhar, K., Ben-Eliyahu, S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. 81 (2), 254-262 (2001).
- Laue, T., et al. Altered NK cell function in obese healthy humans. BMC Obes. 2, 1(2015).
- Hazeldine, J., Lord, J. M. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res Rev. 12 (4), 1069-1078 (2013).
Access restricted. Please log in or start a trial to view this content.
Erratum
Formal Correction: Erratum: A Flow Cytometry-Based Cytotoxicity Assay for the Assessment of Human NK Cell Activity
Posted by JoVE Editors on 9/10/2017. Citeable Link.
An erratum was issued for: A Flow Cytometry-Based Cytotoxicity Assay for the Assessment of Human NK Cell Activity. Figure 4 has been corrected to show background-corrected data.
Figure 4 was updated from:
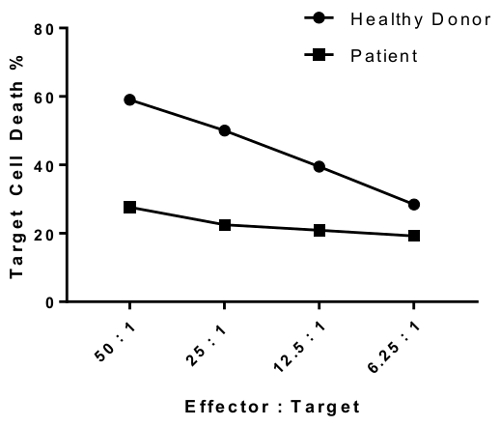
to:
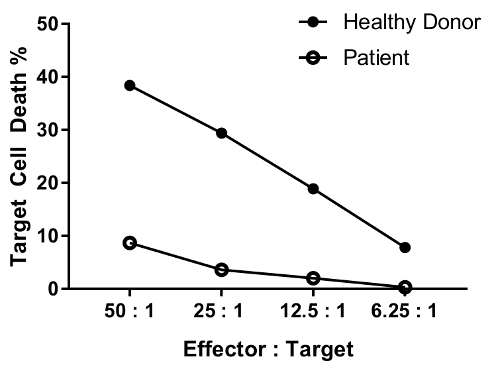
Ristampe e Autorizzazioni
Richiedi autorizzazione per utilizzare il testo o le figure di questo articolo JoVE
Richiedi AutorizzazioneThis article has been published
Video Coming Soon