É necessária uma assinatura da JoVE para visualizar este conteúdo. Faça login ou comece sua avaliação gratuita.
Method Article
Um ensaio de citotoxicidade baseados em citometria de fluxo para a avaliação da atividade das células NK humana
Neste Artigo
Erratum Notice
Resumo
Um método baseado em citometria de fluxo para determinar quantitativamente a atividade citotóxica de células humanas de assassino natural é mostrado aqui.
Resumo
Dentro do sistema imunológico inato, linfócitos efetores conhecidos como natural killer (NK) células desempenham um papel essencial na defesa do hospedeiro contra células aberrantes, eliminando especificamente tumoral e Viralmente infectado as células. Aproximadamente 30 defeitos conhecidos monogénicas, juntamente com uma série de outras condições patológicas, causam ou funcional ou clássica NK célula deficiência, manifestando-se na reduzida ou ausente atividade citotóxica. Historicamente, a citotoxicidade foi investigada com métodos radioativos, que são complicados, caros e potencialmente perigosos. Este artigo descreve um método baseado em citometria de fluxo aerodinâmico, clinicamente aplicável para quantificar a atividade citotóxica das células NK. Neste ensaio, células mononucleares de sangue periférico (PBMC) ou purificadas preparações de células NK são co incubadas em diferentes proporções com uma linhagem de células de tumor alvo conhecida por ser sensível a citotoxicidade mediada por células do NK (NKCC). As células alvo são previamente rotuladas com uma tintura fluorescente para permitir sua discriminação das células efetoras (células NK). Após o período de incubação, as células alvo morto são identificadas por uma mancha de ácido nucleico, que permeia especificamente as células mortas. Este método é passível de diagnóstico e aplicações de pesquisa tanto, graças às capacidades multi-parâmetros de citometria de fluxo, tem a vantagem adicional de potencialmente permitindo uma análise mais profunda do fenótipo de células NK e função.
Introdução
Assassino naturais (NK) células são um subconjunto de sofisticada de linfócitos inatos humanos criticamente envolvidos na eliminação de células infectadas Viralmente, células transformadas e outras ameaças patogênicos 1,2. Grânulos líticas de célula NK citotóxicas proteínas, tais como a perforina e granzymes de casa. Após a ativação, células NK formam uma complexa interação com seus alvos conhecido como sinapse imunológica, através do qual estas moléculas citolíticas localmente são liberadas, resultando na lise de célula alvo direto e apoptose, juntamente com a liberação de citocinas e chemokine e, finalmente, na indução de um inflamatório estado 1,3,4.
Ativação de células NK envolve uma complexa cadeia de ativando e inibitórias interações entre NK celulares receptores e ligantes expressados na superfície das células alvo, formando um sistema rigidamente regulamentado. Um dos mais estudados mecanismos de ativação de células NK é o self"desaparecido". Na verdade, falta de detecção de classe que eu major complexo de histocompatibilidade (MHC), ou moléculas de antigénios (HLA) de leucócitos humanos, na infectado ou transformado a citotoxidade de células NK de células gatilhos. Tumor e células infectadas por vírus geralmente downregulate estes antígenos para escapar a imunidade mediada por células T, tornando-se assim o principal NK células alvos 1,3,4.
Avaliação da função de célula NK principalmente é categorizada em degranulação ou citotoxicidade ensaios. No entanto, ensaios de degranulação, como detecção de fluxo cytometric do marcador degranulação associada CD107a, são apenas indicativos de ativação de células NK e não de sua função final, o abate direto de alvo células 5,6,7,8. Portanto, essa limitação tem atraído investigadores ensaios de citotoxicidade como uma alternativa mais revelador e mais direta.
O velho "padrão ouro" para avaliar a atividade citotóxica mediada por células de células T e NK tanto é o ensaio de liberação de cromo (CRA). CRA envolve radioactivamente rotulagem das células alvo com 51Cr e co, incubando-os com células efetoras. Este ensaio é rica em princípio que esse lysis da pilha resulta na liberação de proteínas 51Cr para o sobrenadante, que pode ser medido pela contagem de gama. Neste ensaio, ao mesmo tempo eficaz, é problemático para uma variedade de razões: elevados custos de material, manipulação e eliminação de radioativo 51Cr, liberação espontânea de 51Cr e difícil padronização - tornando-se completamente impraticável 9,10.
Um número de ensaios não-radioativo, envolvendo rotulagem fluorescente, liberação de enzima e bioluminescência mesmo, desde que foram desenvolvido como alternativas ao CRA 11,12,13,14. Descrevemos aqui um método baseado em citometria de fluxo para medição de NK célula atividade citotóxica sobre células-alvo K562 que é simples, sensível e reprodutível. Células k562 são eu e expressão aumentada de ligantes para receptores NK activatory, que os torna particularmente suscetível a de citotoxicidade mediada por células NK 15, uma célula humana erythroleukemic linha com reduzida expressão da classe HLA. Neste ensaio, células K562 pre-são rotuladas com carboxyfluorescein diacetato succinimidil éster (CFSE) e co cultivadas em várias relações com qualquer células mononucleares de sangue periférico (PBMC) ou purificado de células NK 1. CFSE é um corante fluorescente estável, proteína ligadora que permite a discriminação das células alvo do efetor NK células 16,17. Após a incubação co, uma mancha de ácido nucleico, especificamente que permeiam a membrana das células mortas, é usada para identificar as células alvo morto (veja a tabela de materiais). As amostras são então adquiridas em um citômetro de fluxo para determinar a percentagem de mortos (i.e., mancha +) CFSE + nas células-alvo.
Este ensaio pode ser usado como uma rotina diagnóstica triagem para defeitos monogénicas afetando o compartimento de célula NK, que são aproximadamente 30 defeitos conhecidos causando funcional ou clássica deficiência de células NK, e para hemophagocytosis hemofagocítica primário ou secundário. Também é útil investigar a atividade das células NK em pacientes com infecções virais herpes recorrente, grave, para avaliar a reconstituição imunológica após transplante de células hematopoiéticas ou post imuno terapia 18,19,20e para uma série de aplicações de pesquisa básica.
Access restricted. Please log in or start a trial to view this content.
Protocolo
Samples were collected according to the ethical guidelines established by the UCLA Human Research Protection Program and IRB approved.
1. Preparation of reagents
NOTE: Unless otherwise stated, all reagents should be allowed to equilibrate at room temperature prior to use. All reagents must remain sterile.
- Prepare a 2x working solution of Tween-20 (i.e., 0.2%) by adding 10 µL of Tween-20 solution to 5 mL of phosphate-buffered saline (PBS) without calcium and magnesium with a p20 pipette.
- Given the high viscosity of Tween-20, take the following steps to ensure accuracy: collect slowly to avoid bubble formation, do not submerge the entire tip to avoid carry-over, and pipette up and down several times in PBS to wash out all Tween-20 from the tip.
- Add 2 µL of IL-2 stock solution (2.1E6 IU/mL) to 198 µL of complete media (i.e., 1:100 dilution), which is RPMI with 1% penicillin-streptomycin and 10% fetal bovine serum (FBS), then proceed to an additional 1:100 dilution by adding 2 µL of IL-2 intermediate solution to 198 µL of complete media to prepare a 7x working solution (210 IU/mL).
- Add 2 µL of CFSE stock solution (5 mM) to 500 µL of plain media (RPMI). Vortex, spin down to remove drops from the cap, then proceed with an additional 1:20 dilution by adding 50 µL of the CFSE intermediate solution to 950 µL of plain RPMI to obtain a 2x working solution of 1 µM. Vortex well and keep protected from light.
2. Isolation of PBMCs as effector cells
NOTE: This assay has been validated for effective use with total PBMCs from healthy controls. However, it is recommended that NK cell content be verified with each PBMC preparation (Figure 1). Also, the volume of whole blood for collection is based on the frequency of NK cells in peripheral whole blood and this may vary from person to person, particularly between healthy donors and patients.
- Collect a minimum of 4 mL of human whole blood in sodium or lithium heparinized blood collection tubes. Store blood samples at room temperature upon collection and use within 30 h of collection.
- Dilute whole blood with 4 mL of PBS (i.e. equal volume of PBS to whole blood).
- Add 4 mL of density gradient solution (see table of materials) to a 15-mL conical tube. Carefully overlay diluted whole blood over the density gradient solution by tilting the tube.
- Centrifuge at 650 x g for 24 min with the brake off.
- Carefully collect the layer of mononuclear cells, which is the thin white layer below the top layer of plasma and platelets, into a new 15-mL conical tube. Bring the volume to 15 mL with PBS.
- Centrifuge at 450 x g for 10 min with the brake on. Aspirate the supernatant and resuspend cells in 0.5 mL of complete media.
- Use an automated cell counter or hemocytometer to count the cells, and adjust the PBMC concentration to 5x106 cells/mL with complete media.
- Check the NK cell content by flow cytometry (see step 4).
- Start the assay within 30 min. Keep cells at room temperature for up to 10 min, or place in a humidified 5% CO2 incubator at 37 °C until ready for use.
3. Isolation of NK cells as effector cells
NOTE: This portion of the protocol is an alternative to using total PBMCs as effector cells. The typical yield from 4 mL of whole blood from a healthy individual is approximately 4x105 NK cells, or approximately 5-15% of PBMCs, though this frequency varies between donors 21. It is recommended that a purity check be performed after the isolation.
- Collect a minimum of 4 mL of human whole blood in sodium or lithium heparinized blood collection tubes. Take 50 µL of whole blood to stain as a pre-enrichment sample.
- Add the enrichment cocktail to blood samples at 50 µL/mL (see table of materials). Mix well and incubate for 10 min at room temperature.
- Add an equal volume of PBS + 2% FBS to dilute the sample and gently mix well.
- Add 4 mL of density gradient solution to a 15-mL conical tube. Carefully overlay the diluted sample on top of the density gradient medium.
- Centrifuge at 1,200 x g for 10 min with the brake off.
- Carefully collect the layer of enriched cells into a new 15-mL conical tube. Wash the cells by bringing the volume up to 50 mL with PBS + 2% FBS.
- Centrifuge at 300 x g for 10 min. Discard the supernatant and repeat the wash.
- Aspirate the supernatant and resuspend cells in 0.5 mL of complete media.
- Using an automated cell counter or hemocytometer, count the cells and adjust the concentration to 5x105 cells/mL. Take 50 µL of purified cell suspension to stain as a post-enrichment sample.
- Keep cells at room temperature for up to 10 min, or place in a humidified 5% CO2 incubator at 37 °C until ready for use.
4. Immunophenotyping for assessment of NK cell content
- Using 50 µL total isolated PBMCs, pre-enrichment whole blood, or purified NK cells, stain with anti-CD56 fluorochrome-conjugated antibody as an NK cell marker. Markers for other cell types and lineages (i.e. CD3+ T cells, CD19+ B cells) may also be included to assess the origin of contamination, if any.
- Incubate for 20 min at 4 °C, and wash by adding 2 mL of PBS + 2% FBS.
- Centrifuge at 450 x g for 5 min.
- For PBMCs or NK cells, aspirate supernatant and resuspend cells in 100 µL of PBS + 2% FBS.
- For whole blood samples, perform red blood cell (RBC) lysis:
- After centrifugation, aspirate the supernatant and resuspend in 2 mL red blood cell lysis buffer (31.75 g of ammonium chloride + 4.16 g of Tris base + 6.65 g of EDTA, dissolved in 5000 mL of deionized water).
- Incubate for 10 min at room temperature.
- Repeat wash twice, aspirate the supernatant, and resuspend lysed sample in 100 µL of PBS + 2% FBS.
NOTE: RBC lysis may also be performed prior to staining. - Proceed with flow cytometric analysis.
5. Thawing, culturing, and harvesting of K562 target cells
- Aliquot 10 mL of complete media into a 15-mL conical tube and allow to warm in a 37 °C water bath. For the thawing and culturing of K562 cells, use only media pre-warmed to 37 °C for all steps.
- Place one vial of frozen K562 cells in the 37 °C water bath until most of it is thawed.
- Transfer the semi-thawed K562 cells to the complete media, pre-warmed to 37 °C, in the 15-mL conical tube. Wash the inside of the cryogenic vial with some pre-warmed media, and add the wash to the 15-mL tube.
- Centrifuge at 140 x g for 7 min with the brake on. Remove the supernatant and resuspend the pellet in 1 mL of complete media.
- Aliquot an appropriate amount of cell suspension to count cells using an automated cell counter or hemocytometer.
- Adjust the concentration to 1x105-2x105 cells/mL and culture in a humidified 5% CO2 incubator at 37 °C for at least 2 days before use. Maintain the culture at this density range for no longer than 3 months, then thaw a new aliquot. Never exceed a cell concentration of 1x106 cells/mL in culture.
- For use in the assay, mix the K562 cell culture well with a 10-mL serological pipette and remove an aliquot to count and assess cell viability using an automated cell counter or hemocytometer and Trypan Blue staining.
- If the viability is less than 85%, proceed with the following dead cell removal steps.
- Add 15 mL of density gradient solution to 50-mL conical tubes, as needed. Collect all cells from culture and slowly overlay the cell suspension over the density gradient solution by tilting the tube.
- Centrifuge at 650 x gfor 24 min with brake off. Carefully collect the layer of live cells into a new 15-mL conical tube. Bring the volume to 50 mL with PBS.
- Centrifuge at 450 x g for 10 min with the brake on. Remove the supernatant and resuspend the pellet in 1 mL of complete media. Count the cells and check cell viability (as in 5.7).
- Culture in a humidified 5% CO2 incubator at 37 °C for at least 2 days before use.
- If the viability is less than 85%, proceed with the following dead cell removal steps.
- Collect 5x105 K562 target cells and centrifuge at 140 x g for 7 min. Aspirate the supernatant and resuspend the cell pellet in 500 µL in media with 1% FBS.
6. Labeling of K562 target cells
NOTE: Make sure the K562 cells are well resuspended before adding the CFSE working solution.
- Pipette 500 µL of the CFSE working solution to the 500 µL of K562 cells for a final concentration of 0.5 µM. Using the same 1000-1250 µL tip, mix immediately by gently pipetting up and down 3-5 times.
- Incubate in the 37 °C water bath or in a humidified 5% CO2 incubator at 37 °C for 10 min in the dark.
NOTE: Since the temperature equilibration lag time is longer in the incubator compared to the water bath, incubation time might need to be adjusted especially for volumes > 1 mL. - Quench the labeling reaction with 10 mL of complete media for 10 min in the dark at room temperature.
- Centrifuge the labeled K562 cells at 140 x g for 7 min. Remove the supernatant and resuspend in 1 mL of complete media.
- Count the cells using an automated cell counter or hemocytometer and bring the concentration to 1x105 cells/mL in complete media.
- Place cells in a humidified 5% CO2 incubator at 37 °C until ready for use. Cells can be kept at room temperature for up to 10 min, or in in a humidified CO2 incubator at 37 °C until ready for use. Do not refrigerate.
7. Plating
NOTE: If NK cells are limiting, the concentration of both K562 and NK cells can be halved, so that half of the cells are plated in the assay. The assay yields comparable results with half the amount of cells (as long as the ratios are kept consistent).
- If using total PBMCs, label a 96-well, U-bottom, tissue culture-treated plate for the following conditions per well: (1) Effector (E) : Target (T) = 50 : 1 + IL-2 - positive control for NK cell cytotoxicity; (2) E : T = 50 : 1; (3) E : T = 25 : 1; (4) E : T = 12.5 : 1; (5) E : T = 6.25 : 1; (6) T - target cells only - negative control for K562 death; (7) E - effector cells only; (8) T + Tween - Positive control for K562 death
- If using purified NK cells, label a 96-well, U-bottom, tissue culture treated plate for the following conditions per well: (1) Effector (E) : Target (T) = 5: 1 + IL-2 - positive control for NK cell cytotoxicity; (2) E : T = 5 : 1; (3) E : T = 2.5 : 1; (4) E : T = 1.25 : 1; (5) E : T = 0.625: 1; (6) T - target cells only - negative control for K562 death; (7) E - effector cells only; (8) T + Tween - positive control for K562 death
- Add 100 µL of complete media to conditions 3, 4, 5, 6, 7. For this and all subsequent steps, (recommended) use a multichannel pipette.
- Add 100 µL of effector cells (PBMCs at 5x106 cells/mL or purified NK cells at 5x105 cells/mL) to conditions 1, 2, 3. Add effector cells for conditions 4-7 by serial dilution. Do not change tips from condition to condition.
- Mix condition 3, take 100 µL, and transfer to condition 4.
- Mix condition 4, take 100 µL, and transfer to condition 5.
- Mix condition 5, take 100 µL, and transfer to condition 7; Condition 6 does not have effector cells.
- Add 100 µL of prepared 2x 0.2% Tween working solution to condition 8 (final concentration: 0.1% Tween-20); condition 8 does not have effector cells.
- Add 30 µL of IL-2 working solution to condition 1. IL-2 should be added to the effector cell suspension prior to the addition of target cell suspension (final concentration: 27 IU/mL).
- Add 100 µL of target cells (labeled K562 cells at 1x105 cells/mL) to conditions 1, 2, 3, 4, 5, 6, 8. Condition 7 does not have target cells.
- Mix all wells with a multichannel pipette and centrifuge the plate at 120 x g for 2 min.
- Incubate in a humidified CO2 incubator at 37 °C for 4 h. At the end of the incubation, place the plate on ice and proceed to viability staining within 30 min.
8. Staining for viability and acquisition
- Prepare dead cell stain (see table of materials) by adding 3 µL of the 5 µM stock solution to 600 µL of PBS.
- Add 50 µL of diluted dead cell stain to each well for a final volume of 250 µL and a final concentration of 0.005 µM. Mix well with a multichannel pipette.
- Proceed with flow cytometric analysis. Using a flow cytometer, collect at least 1,000 events in the target cell gate (Figure 2) for statistically meaningful results.
Access restricted. Please log in or start a trial to view this content.
Resultados
Antes de configurar o ensaio, é altamente recomendável que o conteúdo da célula NK ser avaliadas na população efetora de escolha. A Figura 1 mostra uma típica coloração de CD56 antes (luz azul) e depois o enriquecimento de célula NK (vermelho). Células NK compreendem até 15% de PBMC e devem ser pelo menos 80% puro depois de enriquecimento.
Análise de fluxo cytometric neste ensaio envolv...
Access restricted. Please log in or start a trial to view this content.
Discussão
O método descrito aqui fornece uma alternativa simples e econômica para o ensaio de libertação tradicionais 51Cr para avaliar a atividade citotóxica das células NK. Esse método é menos demorado do que os métodos padrão anteriores, como CRA, sensível e reprodutível e pode ser usado para ambos clínicos e aplicações de pesquisa.
Enquanto o ensaio funciona com totais PBMCs e enriquecido de células NK, a opção de usar PBMCs sem a necessidade de purificar a populações ...
Access restricted. Please log in or start a trial to view this content.
Divulgações
Os autores não declaram nenhum conflito de interesse financeiro.
Agradecimentos
Gostaríamos de agradecer a Jill Narciso, centro de Imunogenética de UCLA, pela sua assistência com a preparação do manuscrito.
Access restricted. Please log in or start a trial to view this content.
Materiais
| Name | Company | Catalog Number | Comments |
| Phosphate-buffered Saline (1x, w/o Ca2+ and Mg2+) | Corning (Cellgro) | 21-040-CM | |
| Ficoll-Paque PLUS | GE Healthcare | 17-1440-02 | |
| Tween-20 | Sigma | BP337-100 | |
| RPMI 1640 Media | Corning (Cellgro) | 10-040-CV | |
| Heat-inactivated Fetal Bovine Serum | Omega Scientific | FB-02 | |
| Penicillin Streptomycin | Life Technologies | 15140-163 | Stock solution at 10,000 U/mL |
| IL-2 | R&D Systems | 202-IL-050 | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA with BSA as a carrier protein. Reconstitute with 500 ul at 100 μg/mL in sterile 100 mM Acetic Acid containing at least 0.1% bovine serum albumin (2.1x10E6 IU/ml) |
| K562 Cells | ATCC | CCL-243 | Cancer cell line |
| T-75 cell culture flasks | Corning | 431464 | |
| CFSE cell proliferation kit | Life Technologies (CellTrace) | C34554 | Reconstitute I vial with 18 ul DMSO to prepare a 5mM stock solution. Do not freeze/thaw. |
| Sytox Red | Life Technologies | S34859 | Stock solution is provided at 5 μM in 1 mL DMSO. The DMSO solution may be subjected to multiple freeze-thaw cycles without reagent degradation. |
| Sodium/lithium heparin blood collection tubes | BD | 02-687-95 | |
| U-bottom 96-well plate | Corning | CLS3897 | |
| Serological pipettes | BD Falcon | ||
| Polystyrene round-bottom tubes (5mL) | BD Falcon | 14959-5 | |
| 50 mL polypropylene conical tube | BD Falcon | 352070 | |
| 15 mL polypropylene conical tube | BD Falcon | 352097 | |
| Reagent reservoir | USA Scientific | 2321-2230 | |
| Human NK cell enrichment cocktail | StemCell Technologies (RosetteSep) | 15065 |
Referências
- Iannello, A., Debbeche, O., Samarani, S., Ahmad, A. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J Leukoc Biol. 84 (1), 1-26 (2008).
- Caligiuri, M. A. Human natural killer cells. Blood. 112 (3), 461-469 (2008).
- Topham, N. J., Hewitt, E. W. Natural killer cell cytotoxicity: how do they pull the trigger? Immunology. 128 (1), 7-15 (2009).
- Warren, H. S., Smyth, M. J. NK cells and apoptosis. Immunol Cell Biol. 77 (1), 64-75 (1999).
- Tognarelli, S., Jacobs, B., Staiger, N., Ullrich, E. Flow Cytometry-based Assay for the Monitoring of NK Cell Functions. J Vis Exp. (116), (2016).
- Somanchi, S. S., McCulley, K. J., Somanchi, A., Chan, L. L., Lee, D. A. A Novel Method for Assessment of Natural Killer Cell Cytotoxicity Using Image Cytometry. PLoS One. 10 (10), e0141074(2015).
- Alter, G., Malenfant, J. M., Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 294 (1-2), 15-22 (2004).
- Atkinson, E. A., Gerrard, J. M., Hildes, G. E., Greenberg, A. H. Studies of the mechanism of natural killer (NK) degranulation and cytotoxicity. J Leukoc Biol. 47 (1), 39-48 (1990).
- Kim, G. G., Donnenberg, V. S., Donnenberg, A. D., Gooding, W., Whiteside, T. L. A novel multiparametric flow cytometry-based cytotoxicity assay simultaneously immunophenotypes effector cells: comparisons to a 4 h 51Cr-release assay. J Immunol Methods. 325 (1-2), 51-66 (2007).
- Kane, K. L., Ashton, F. A., Schmitz, J. L., Folds, J. D. Determination of natural killer cell function by flow cytometry. Clin Diagn Lab Immunol. 3 (3), 295-300 (1996).
- Jang, Y. Y., et al. An improved flow cytometry-based natural killer cytotoxicity assay involving calcein AM staining of effector cells. Ann Clin Lab Sci. 42 (1), 42-49 (2012).
- Korzeniewski, C., Callewaert, D. M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 64 (3), 313-320 (1983).
- Karimi, M. A., et al. Measuring cytotoxicity by bioluminescence imaging outperforms the standard chromium-51 release assay. PLoS One. 9 (2), e89357(2014).
- Oppenheim, D. E., et al. Glyco-engineered anti-EGFR mAb elicits ADCC by NK cells from colorectal cancer patients irrespective of chemotherapy. Br J Cancer. 110 (5), 1221-1227 (2014).
- West, W. H., Cannon, G. B., Kay, H. D., Bonnard, G. D., Herberman, R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 118 (1), 355-361 (1977).
- Jedema, I., van der Werff, N. M., Barge, R. M., Willemze, R., Falkenburg, J. H. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood. 103 (7), 2677-2682 (2004).
- Lecoeur, H., Fevrier, M., Garcia, S., Riviere, Y., Gougeon, M. L. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J Immunol Methods. 253 (1-2), 177-187 (2001).
- Carotta, S. Targeting NK Cells for Anticancer Immunotherapy: Clinical and Preclinical Approaches. Front Immunol. 7, 152(2016).
- Mandal, A., Viswanathan, C. Natural killer cells: In health and disease. Hematol Oncol Stem Cell Ther. 8 (2), 47-55 (2015).
- Rezvani, K., Rouce, R. H. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front Immunol. 6, 578(2015).
- Angelo, L. S., et al. Practical NK cell phenotyping and variability in healthy adults. Immunol Res. 62 (3), 341-356 (2015).
- Zons, P., et al. Comparison of europium and chromium release assays: cytotoxicity in healthy individuals and patients with cervical carcinoma. Clin Diagn Lab Immunol. 4 (2), 202-207 (1997).
- Yovel, G., Shakhar, K., Ben-Eliyahu, S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. 81 (2), 254-262 (2001).
- Laue, T., et al. Altered NK cell function in obese healthy humans. BMC Obes. 2, 1(2015).
- Hazeldine, J., Lord, J. M. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res Rev. 12 (4), 1069-1078 (2013).
Access restricted. Please log in or start a trial to view this content.
Erratum
Formal Correction: Erratum: A Flow Cytometry-Based Cytotoxicity Assay for the Assessment of Human NK Cell Activity
Posted by JoVE Editors on 9/10/2017. Citeable Link.
An erratum was issued for: A Flow Cytometry-Based Cytotoxicity Assay for the Assessment of Human NK Cell Activity. Figure 4 has been corrected to show background-corrected data.
Figure 4 was updated from:
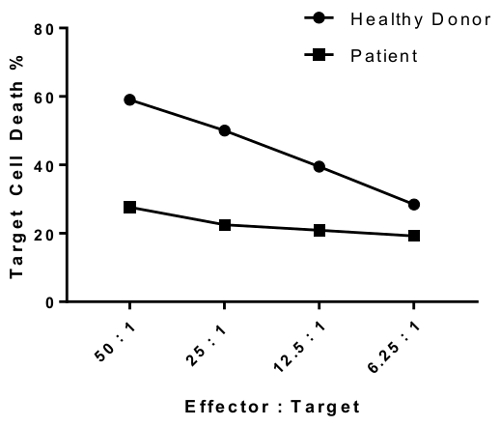
to:
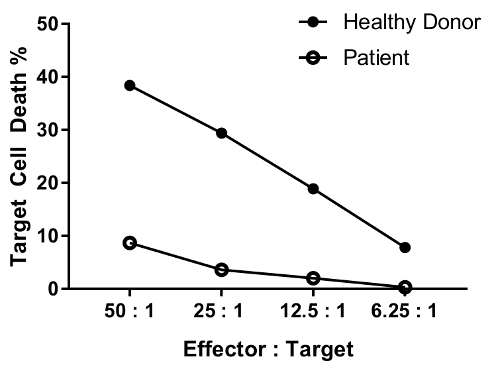
Reimpressões e Permissões
Solicitar permissão para reutilizar o texto ou figuras deste artigo JoVE
Solicitar PermissãoThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Todos os direitos reservados