药物注射 III
Overview
来源: 凯斯图尔特, RVT, RLATG, CMAR;瓦莱丽. 施罗德, RVT, RLATG。圣母大学, 在
在实验室小鼠和大鼠中有许多常用的复合用药路线。然而, 某些协议可能需要使用不太常用的路线, 包括皮内、鼻腔和颅内注射。专业培训对于这些程序的成功执行至关重要。为获得机构动物保育和使用委员会 (IACUC) 批准, 可能需要提供这些路线的理由。
Procedure
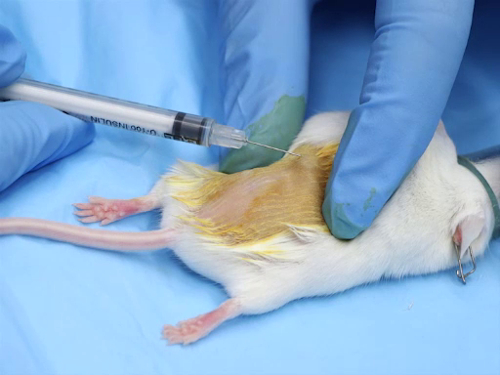
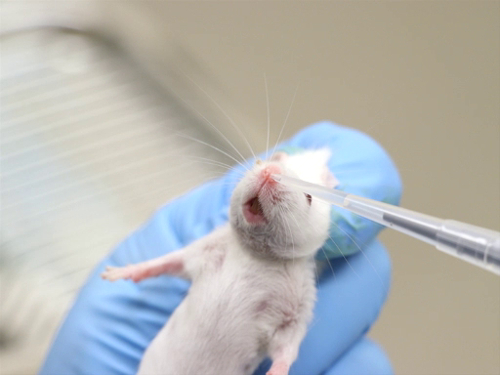
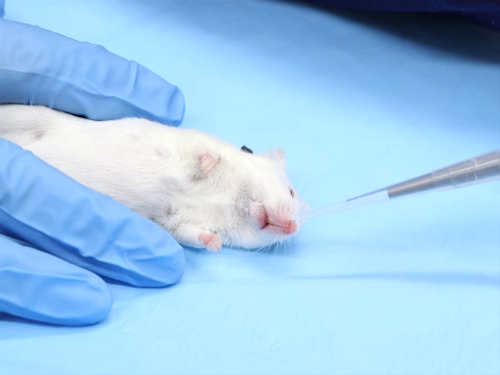
1. 皮内管理
- 大多数皮内注射是基化合物。解决方案必须在生理学上进行缓冲, 以获得中性的 pH 值, 以避免注射部位出现组织坏死.
- 针的大小范围是25-30 计, 最小的可能.
- 每个注射场的剂量范围是50-100 和 #181; 注射过量可能导致注射部位坏死, 或由于压力而在现场漏出该化合物.
- 为了准确地将针放置到皮内空间, 必须对小鼠和老鼠进行麻醉。吸入麻醉可以快速诱导和恢复;然而, 注射麻醉的好处是提供充足的时间来准备该地区和执行注射。 1
- 通过使用脱毛的奶油或通过剃须区域来删除注射部位的毛发。
- 彻底清除任何残留的脱毛膏或毛发碎片.
- 应用外用防腐剂, 如碘溶液、洗必泰溶液或酒精.
- 管理过程
- 在拇指和食指之间拉伸皮肤绷紧。这为皮肤定位针时提供了稳定性.
- 将针锥放在皮肤上.
- 轻轻地将针插入表皮和真皮之间的皮肤。前进的针刚刚超越斜面.
- 慢慢
Application and Summary
References
- Turner, P.V., Pekow, C., Vasbinder, M. A., and Brabb, T. 2011. Administration of substances to laboratory animals: equipment and considerations, vehicle selection, and solution preparation. JAALAS. 50: 614-627.
- Dhuria, S.V., Hanson, L.R., and Frey II, W.H. 2010. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. Journal of Pharmaceutical Sciences. 99: 1654-1673.
- Stevens, J., Suidgeest, E, Van der Graaf, P.H., Danhof, M., and De Lange, E.C. 2008. Development and evaluation of a new, minimal-stress animal model for intranasal administration in freely moving rats. Poster presentation at American Association of Pharmaceutical Scientists Annual Meeting, Atlanta, Georgia.
- Morton, D.A., Jennings, M., Buckwell, A., Ewbank, R., Godfrey, C., Holgate, B., Inglis, I., James, R., Page, C., Sharman, I., Verschoyle, R., Westall, L., and Wilson, A.B. 2001. Refining procedures for the administration of substances Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Members of the Joint Working Group on Refinement. Laboratory Animals. 35: 1-41.
Tags
跳至...
此集合中的视频:

Now Playing
药物注射 III
Lab Animal Research
31.4K Views

啮齿动物的处理和克制技术
Lab Animal Research
174.4K Views

基本护理程序
Lab Animal Research
28.0K Views

育种和断奶的基本原理
Lab Animal Research
35.7K Views

啮齿动物识别 I
Lab Animal Research
54.7K Views

啮齿动物鉴定 (2)
Lab Animal Research
25.6K Views

药物注射 I
Lab Animal Research
100.5K Views

药物注射 II
Lab Animal Research
34.9K Views

药物注射 IV
Lab Animal Research
51.6K Views

血液采集 I
Lab Animal Research
171.5K Views

血液采集 II
Lab Animal Research
73.2K Views

麻醉诱导与维护
Lab Animal Research
50.4K Views

啮齿动物手术的考虑事项
Lab Animal Research
22.5K Views

诊断尸检和组织采集
Lab Animal Research
58.0K Views

无菌组织采集
Lab Animal Research
34.8K Views
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。