11.10 : Sharpless-Epoxidierung
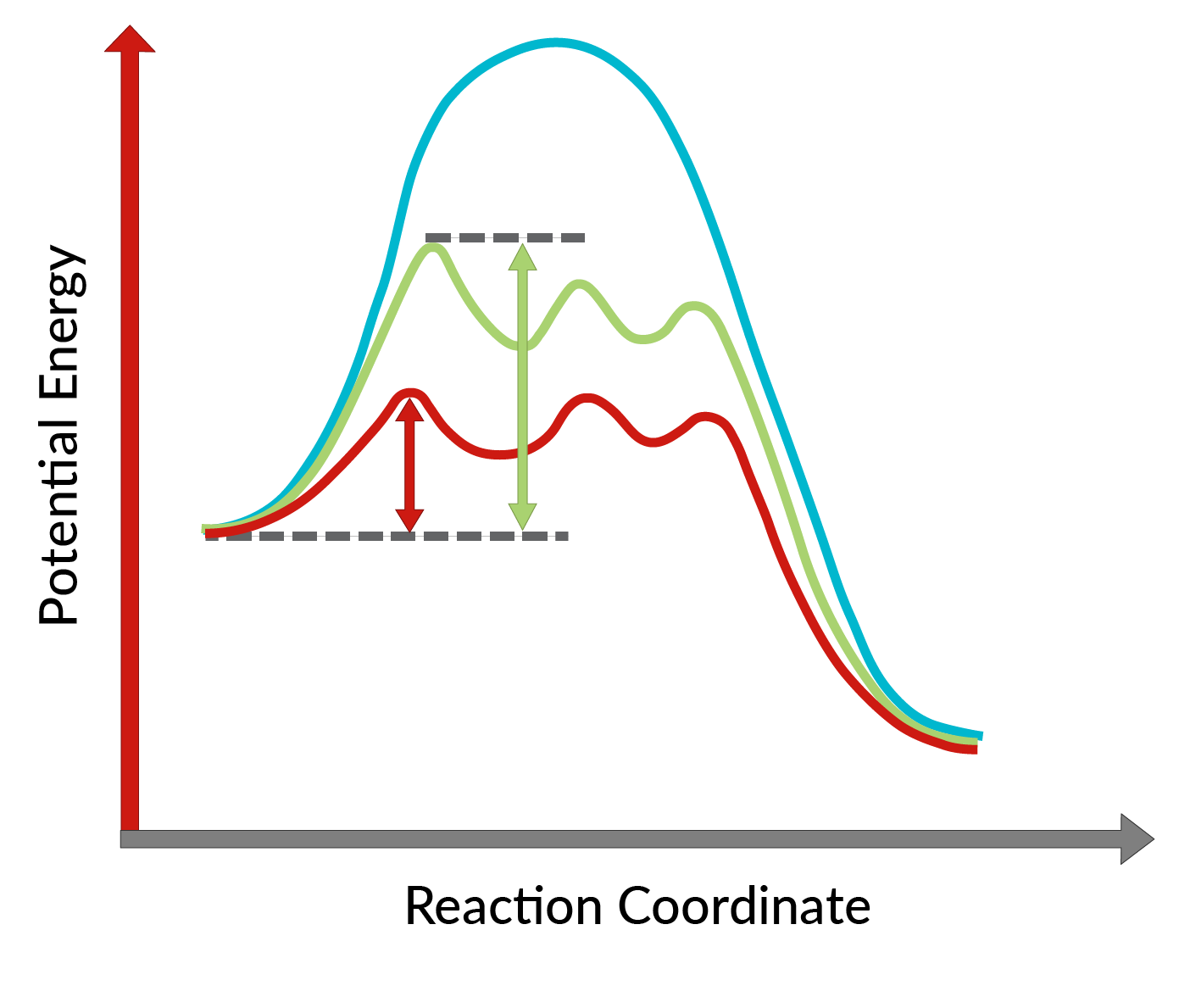
Die Umwandlung von Allylalkoholen in Epoxide unter Verwendung eines chiralen Katalysators wurde von K. Barry Sharpless entdeckt und ist als Sharpless-Epoxidierung bekannt. Die Verwendung eines chiralen Katalysators ermöglicht die Bildung eines Enantiomers des Produkts im Überschuss. Bei diesem chiralen Katalysator handelt es sich hauptsächlich um einen chiralen Komplex aus Titantetraisopropoxid und Tartratester (spezifisches Stereoisomer). Das im chiralen Katalysator verwendete Stereoisomer bestimmt die Bildung des Enantiomers des Produkts. Mit anderen Worten, die Verwendung von L-(+)-Diethyltartrat führt zu Enantiomeren mit dem Epoxidring unterhalb der Ebene, während mit D-(-)-Diethyltartrat Enantiomere mit dem Epoxidring oberhalb der Ebene entstehen. Die hohe Enantioselektivität der Reaktion lässt sich erklären, wenn man die Aktivierungsenergie betrachtet, die erforderlich ist, damit die Reaktion in Gegenwart des chiralen Katalysators in Vorwärtsrichtung abläuft. Wie in Abbildung 1 dargestellt, sinkt die Aktivierungsenergie der Reaktion im Vergleich zur unkatalysierten Reaktion (blaue Kurve) mit der Zugabe des chiralen Katalysators drastisch (rote und grüne Kurve). Außerdem wird die Aktivierungsenergie für die Bildung eines Enantiomers (rote Kurve) stärker gesenkt als die eines anderen Enantiomers (grüne Kurve), was zur Bildung eines Enantiomers im Überschuss führt. Daher kann die Sharpless-Epoxidierungsreaktion für die Synthese der gewünschten Enantiomere des Produkts verwendet werden.
Die Stereochemie des Produkts, das bei der Sharpless-Epoxidierung eines beliebigen Allylalkohols entsteht, lässt sich vorhersagen, indem man das Allylalkoholmolekül einfach in einer Ebene ausrichtet, wobei die Hydroxylgruppen in die untere rechte Ecke zeigen, wie in Abbildung 2 dargestellt. In dieser planaren Struktur liefert D-(-)-Diethyltartrat den Sauerstoff von der Oberseite des Alkens, so dass die Epoxidbildung von oberhalb der Ebene aus möglich ist, während L-(+)-Diethyltartrat den Sauerstoff von der Unterseite des Alkens liefert, so dass der Epoxidring von unterhalb der Ebene gebildet wird.
Aus Kapitel 11:

Now Playing
11.10 : Sharpless-Epoxidierung
Ether, Epoxide, Sulfide
3.8K Ansichten

11.1 : Struktur und Nomenklatur der Ether
Ether, Epoxide, Sulfide
11.2K Ansichten

11.2 : Physikalische Eigenschaften von Ethern
Ether, Epoxide, Sulfide
6.9K Ansichten

11.3 : Ether aus Alkoholen: Alkoholdehydrierung und Williamson-Ether-Synthese
Ether, Epoxide, Sulfide
10.2K Ansichten

11.4 : Ether aus Alkenen: Alkoholaddition und Alkoxymerkuration-Demerkuration
Ether, Epoxide, Sulfide
7.7K Ansichten

11.5 : Ether zu Alkylhalogeniden: Saure Spaltung
Ether, Epoxide, Sulfide
5.6K Ansichten

11.6 : Autoxidation von Ethern zu Peroxiden und Hydroperoxiden
Ether, Epoxide, Sulfide
7.4K Ansichten

11.7 : Kronen-Äther
Ether, Epoxide, Sulfide
5.1K Ansichten

11.8 : Struktur und Nomenklatur von Epoxiden
Ether, Epoxide, Sulfide
6.3K Ansichten

11.9 : Herstellung von Epoxiden
Ether, Epoxide, Sulfide
7.4K Ansichten

11.11 : Säurekatalysierte Ringöffnung von Epoxiden
Ether, Epoxide, Sulfide
7.1K Ansichten

11.12 : basenkatalysierte Ringöffnung von Epoxiden
Ether, Epoxide, Sulfide
8.3K Ansichten

11.13 : Struktur und Nomenklatur von Thiolen und Sulfiden
Ether, Epoxide, Sulfide
4.6K Ansichten

11.14 : Herstellung und Reaktionen von Thiolen
Ether, Epoxide, Sulfide
6.0K Ansichten

11.15 : Herstellung und Reaktionen von Sulfiden
Ether, Epoxide, Sulfide
4.7K Ansichten
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten