20.19 : Joule-Thomson Effect
The Joule-Thomson effect, also known as the Joule-Kelvin effect, describes the temperature change of a fluid when it is forced through a valve or porous plug while keeping it in a thermally insulated environment. This experiment is called a throttling process. This is an important effect widely used in refrigeration and the liquefaction of gases.
This experiment forces high-pressure gas through a throttle valve or a porous plug to a lower-pressure region. The gas expands as it passes through to the low-pressure region and occupies the volume, which is larger than the initial volume. Work is done on the gas when gas is forced through a throttle valve. However, when it expands, work is done by the gas on the surroundings. The temperature of the gas varies in this process; hence, it attains a new internal energy. The change in internal energy of the gas must be equal to the work done on the gas minus the work done by the gas. It is found that enthalpy is conserved (isenthalpic) in this flow process.
The change in temperature of the gas when pressure is reduced at constant enthalpy is given by the Joule-Thomson coefficient:
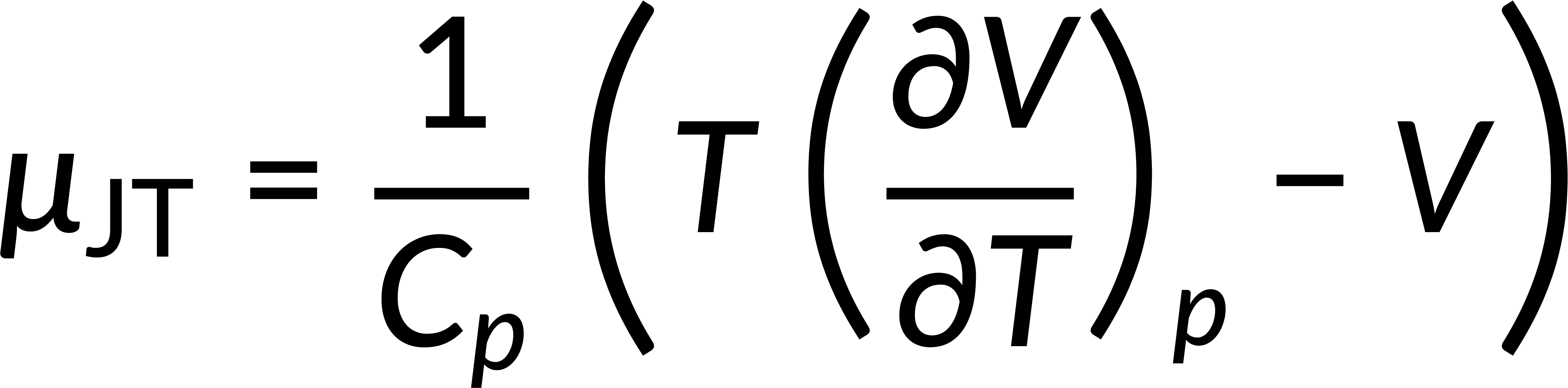
Since the temperature of the gas varies, the Joule-Thomson coefficient can take either sign, depending on whether the gas becomes heated or cooled. The inversion curve in the T-p plane explains the heating and cooling of gas during the throttling process.
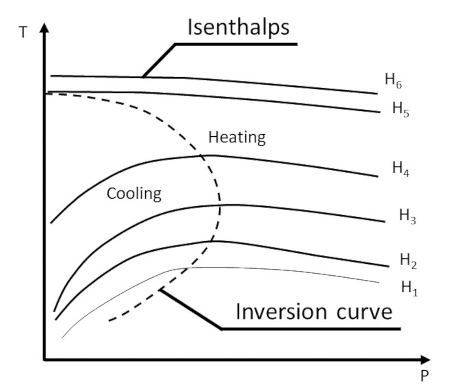
The inversion curve separates the region of heating from the region of cooling. The maxima of the different isenthalps lie on the inversion curve. Note that an isenthalp is the locus of the end points of different throttling processes, starting at the same initial state. Depending on where the initial and final points are chosen, both heating and cooling effects can be produced during the throttling process.
The Joule-Thomson coefficient is zero for an ideal gas, indicating there is no temperature change when an ideal gas undergoes a throttling process. The coefficient is negative for real gases at extremely high and extremely low temperatures.
Aus Kapitel 20:

Now Playing
20.19 : Joule-Thomson Effect
The First Law of Thermodynamics
2.9K Ansichten

20.1 : Thermodynamische Systeme
The First Law of Thermodynamics
4.9K Ansichten

20.2 : Geleistete Arbeit während der Lautstärkeänderung
The First Law of Thermodynamics
3.9K Ansichten

20.3 : Pfad zwischen thermodynamischen Zuständen
The First Law of Thermodynamics
3.0K Ansichten

20.4 : Wärme und freie Ausdehnung
The First Law of Thermodynamics
1.7K Ansichten

20.5 : Innere Energie
The First Law of Thermodynamics
4.5K Ansichten

20.6 : Erster Hauptsatz der Thermodynamik
The First Law of Thermodynamics
4.1K Ansichten

20.7 : Erster Hauptsatz der Thermodynamik: Problemlösung
The First Law of Thermodynamics
2.5K Ansichten

20.8 : Zyklische Prozesse und isolierte Systeme
The First Law of Thermodynamics
2.7K Ansichten

20.9 : Isotherme Prozesse
The First Law of Thermodynamics
3.5K Ansichten

20.10 : Isochore und isobare Prozesse
The First Law of Thermodynamics
3.3K Ansichten

20.11 : Wärmekapazitäten eines idealen Gases I
The First Law of Thermodynamics
2.6K Ansichten

20.12 : Wärmekapazitäten eines idealen Gases II
The First Law of Thermodynamics
2.4K Ansichten

20.13 : Wärmekapazitäten eines idealen Gases III
The First Law of Thermodynamics
2.1K Ansichten

20.14 : Adiabatische Prozesse für ein ideales Gas
The First Law of Thermodynamics
3.0K Ansichten
See More
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten