Complejos de coordinación
Visión general
Fuente: Laboratorio de Dr. Neal Abrams — Universidad de SUNY de la ciencia ambiental y silvicultura
Metales de transición se encuentran en todas partes de suplementos para baños de galvanoplastia. Metales de transición también forman los pigmentos en muchas pinturas y componer todos los minerales. Por lo general, metales de transición se encuentran en la forma catiónica ya que fácilmente se oxida, o pierden electrones y están rodeados de donadores de electrones llamados ligandos. Estos ligandos no no forma iónica o covalentes con centro metálico, algo toman en un tercer tipo de enlace conocido como covalente coordinado. El enlace covalente coordinado entre un metal y un ligando es dinámico, lo que significa que ligandos son continuamente intercambiando y volver a coordinación alrededor del centro de metal. Las identidades del metal y del ligando dicta que ligandos se adherirá preferentemente sobre otro. Además, color y propiedades magnéticas son también debido a los tipos de complejos que se forman. Los compuestos de coordinación que forman son analizados usando una variedad de instrumentos y herramientas. Este experimento explora por qué tantos complejos son posibles y utiliza un método spectrochemical (color y química) para ayudar a identificar el tipo de coordinación complejo que se forma.
Procedimiento
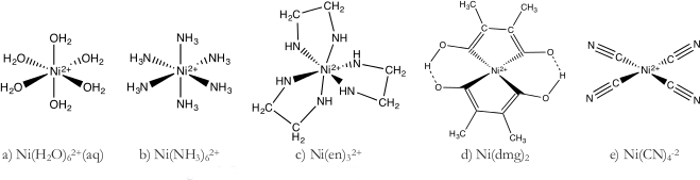
1. níquel complejos y colores
- Ni (H2O)62 + complejo (Figura 1a)
- Preparar una solución de 1 M de Ni (H2O)62 + disolviendo NiSO4 en el volumen adecuado de agua.
- Diluir más la Ni (H2O)62 +solución al agregar 70 mL de solución de 1 M de a 1.000 mL de agua desionizada.
- Dividir el Ni (H2O)62 + entre siete vasos de precipitado
Aplicación y resumen
De los pigmentos a la gente, metales de transición se encuentran en campos de la química, la biología, la geología y la ingeniería. Entender el comportamiento de los metales de transición bajo diferentes Estados químicos puede ser tan simple como el color control o comportamiento magnético. Casi todos metales de transición 3d (4th fila) es vital para la función fisiológica y, en todos los casos, estos metales están sujetos a ligandos para formar complejos de coordinación. Por ejemplo, el hierro es...
Referencias
- Shakhashiri, B. Z.; G. E. Dirreen, G. E; Juergens, F. Color, Solubility, and Complex Ion Equilibria of Nickel (II) Species in Aqueous Solution. J. Chem. Ed. 52 (12), 900-901 (1980).
Tags
Saltar a...
Vídeos de esta colección:

Now Playing
Complejos de coordinación
General Chemistry
91.7K Vistas

Cristalería de laboratorio y usos comunes
General Chemistry
658.8K Vistas

Soluciones y concentraciones
General Chemistry
275.3K Vistas

Determinación de la densidad de un sólido y líquido
General Chemistry
556.8K Vistas

Determinación de la composición porcentual en masa de una solución acuosa
General Chemistry
383.8K Vistas

Determinación de la fórmula empírica
General Chemistry
183.7K Vistas

Determinación de las reglas de solubilidad de compuestos iónicos
General Chemistry
141.6K Vistas

Uso del medidor de pH
General Chemistry
346.9K Vistas

Introducción a la titulación
General Chemistry
425.6K Vistas

Ley del Gas ideal
General Chemistry
79.2K Vistas

Determinación espectrofotométrica de la constante de un equilibrio
General Chemistry
158.8K Vistas

Principio de le Châtelier
General Chemistry
265.8K Vistas

Depresión del punto de congelación para determinar un compuesto desconocido
General Chemistry
160.8K Vistas

Determinación de las leyes de la velocidad y el orden de la reacción
General Chemistry
196.3K Vistas

Uso de la calorimetría diferencial para medir los cambios en la entalpía
General Chemistry
44.8K Vistas
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados