Spectroscopie à résonance magnétique nucléaire (RMN)
Vue d'ensemble
Source : Laboratoire de Dr. Henrik Sundén-Chalmers University of Technology
Spectroscopie de résonance magnétique nucléaire (RMN) est une technique d’analyse essentiel pour les chimistes organiques. Avec l’aide de la RMN, le travail dans le laboratoire biologique a été facilité énormément. Non seulement peut il fournir des informations sur la structure d’une molécule mais également de déterminer la teneur et la pureté d’un échantillon. Par rapport aux autres techniques usuelles pour les chimistes organiques — comme l’analyse thermique et de spectrométrie de masse (MS) — RMN est une méthode non destructive qui est utile lorsque la récupération de l’échantillon est importante.
Une des techniques plus fréquemment utilisées de NMR pour un chimiste organicien est NMR protonique (1H). Les protons présents dans une molécule seront comporte différemment selon son environnement chimique, ce qui permet d’élucider sa structure. En outre, il est possible de surveiller l’achèvement d’une réaction en comparant les spectres de RMN des matières premières à celle du produit final.
Cette vidéo illustre bien comment la spectroscopie RMN peut être utilisée dans le travail quotidien d’un chimiste organicien. S’affichera alors ce qui suit : J’ai) préparation d’un échantillon de NMR. II) à l’aide d' 1H RMN pour surveiller une réaction. III) identifiant le produit obtenu par une réaction avec 1H RMN. La réaction qui sera montrée est la synthèse d’un E- chalcone (3) d’un (1) d’aldéhyde et une cétone (2) (schéma 1). 1
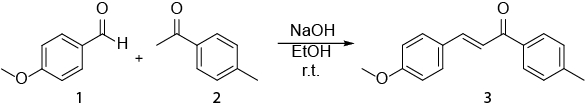
Schéma 1. Synthèse des (2E)-3-(4-methoxyphenyl)-1-(4-methylphenyl)-2-propen-1-one.
Procédure
1. préparation de la RMN, matière première
- Ajouter environ 10 mg à partir de matériel dans un tube propre de NMR.
- Dissoudre le produit de départ dans un solvant deutéré de mL ~0.7 (exemple donné CDCl3). Une hauteur adéquate du solvant pour un bon spectre est de 4,5 à 5 cm.
- Boucher le tube NMR soigneusement et écrire le nom de l’échantillon sur la PAC.
- Agiter l’échantillon doucement pour s’assurer que tout le matériel avait dissous. Prendre soin d’éviter tout contact en
Résultats
En comparant les spectres des produits de départ (Figures 1 et 2) à celle du produit final (Figure 5), une nette différence entre les spectres peut être observée, indiquant la formation de la chalcone. Le point de terminaison de la réaction peut être détermine en prélevant des échantillons de NMR à différents intervalles de temps ; par exemple, le pic de proton aldéhyde (C(=O)H) (1) peut être vu à la
Applications et Résumé
NMR, par exemple, permet de détecter des intermédiaires réactionnels, facilitant le travail dans l’élucidation d’un mécanisme de réaction. Avec l’aide de la RMN, il est également possible d’observer les interactions et les mouvements moléculaires importantes pour le développement de médicaments. En outre, NMR peut donner des informations structurelles sur les matériaux solides ; par exemple pour fournir une justification pour les propriétés matérielles observées. Autres applications de la RMN peuvent être trouvées d...
References
- Ta, L., Axelsson, A., Bijl, J., Haukka, M., Sundén, H., Ionic Liquids as Precatalysts in the Highly Stereoselective Conjugate Addition of α,β-Unsaturated Aldehydes to Chalcones. Chem. Eur. J. 20 (43), 13889-13893 (2014).
- Table adapted from Graham Solomons, T. W. Fryhle, C. B., Organic Chemistry, 10th edition, Wiley, p. 387, 418 (2011).
- Clayden, J., Greeves, N., Warren, S., Wothers, P. Proton nuclear magnetic resonance. Organic Chemistry, Chapter 11, Oxford University Press, 269 (2001).
- Wu, X.-F., Neumann, H., Spannenberg, A., Schulz, T., Jiao, H., Beller, M.,Development of a General Palladium-Catalyzed Carbonylative Heck Reaction of Aryl Halides. J. Am. Chem. Soc. 132 (41), 14596-14602 (2010).
Tags
Passer à...
Vidéos de cette collection:

Now Playing
Spectroscopie à résonance magnétique nucléaire (RMN)
Organic Chemistry
248.0K Vues

Introduction à la catalyse
Organic Chemistry
34.5K Vues

Montage d'un chauffage à reflux
Organic Chemistry
167.6K Vues

Réaliser des réactions en dessous de la température ambiante
Organic Chemistry
70.7K Vues

Transfert de solvants via une rampe à vide (ligne Schlenk)
Organic Chemistry
41.6K Vues

Dégazage des liquides par la technique "cycle geler-pomper-dégeler"
Organic Chemistry
56.1K Vues

Préparation de réactifs anhydres et équipement
Organic Chemistry
79.4K Vues

Purification des composés par recristallisation
Organic Chemistry
708.9K Vues

Séparation des mélanges par précipitation
Organic Chemistry
157.8K Vues

Extraction solide-liquide
Organic Chemistry
237.9K Vues

Utilisation d'un évaporateur rotatif (ou Rotovap) pour éliminer un solvant
Organic Chemistry
212.9K Vues

Distillation fractionnée
Organic Chemistry
334.5K Vues

Préparation de cristaux pour analyse par diffraction des rayons X
Organic Chemistry
32.4K Vues

Performing 1D Thin Layer Chromatography
Organic Chemistry
289.8K Vues

Chromatographie sur colonne
Organic Chemistry
360.3K Vues