Spettroscopia a risonanza magnetica nucleare (NMR)
Panoramica
Fonte: Laboratorio del Dr. Henrik Sundén – Chalmers University of Technology
La spettroscopia di risonanza magnetica nucleare (NMR) è una tecnica di analisi vitale per i chimici organici. Con l'aiuto di NMR, il lavoro nel laboratorio organico è stato facilitato enormemente. Non solo può fornire informazioni sulla struttura di una molecola, ma anche determinare il contenuto e la purezza di un campione. Rispetto ad altre tecniche comunemente incontrate per i chimici organici – come l'analisi termica e la spettrometria di massa (MS) – la NMR è un metodo non distruttivo che è prezioso quando il recupero del campione è importante.
Una delle tecniche NMR più frequentemente utilizzate per un chimico organico è la NMR protonica(1H). I protoni presenti in una molecola si comporteranno in modo diverso a seconda dell'ambiente chimico circostante, rendendo possibile chiarire la sua struttura. Inoltre, è possibile monitorare il completamento di una reazione confrontando gli spettri NMR del materiale di partenza con quelli del prodotto finale.
Questo video esemplifica come la spettroscopia NMR può essere utilizzata nel lavoro quotidiano di un chimico organico. Verrà mostrato quanto segue: i) preparazione di un campione NMR. ii) Utilizzo di 1H NMR per monitorare una reazione. iii) Identificazione del prodotto ottenuto da una reazione con 1H NMR. La reazione che verrà mostrata è la sintesi di un E-calcone (3) da un'aldeide (1) e un chetone (2) (Schema 1). 1
Schema 1. Sintesi di (2E)-3-(4-metossifenil)-1-(4-metilfenil)-2-propen-1-one.
Principi
I nuclei con una massa dispari o un numero atomico avranno una proprietà chiamata spin, rendendo possibile l'uso della NMR per rilevare elementi come idrogeno(1H), carbonio(13C) e fosforo(31P). Gli spin sono casuali e girano in direzioni casuali; tuttavia, applicando un campo magnetico esterno questi nuclei si allineeranno con o contro il campo magnetico applicato. Questi due stati hanno diversi livelli di energia: uno stato a bassa energia e uno stato ad alta energia. L'irradiazione con radiazioni elettromagnetiche farà sì che lo stato a bassa energia si capovolga allo stato ad alta energia. Quando la radiazione è cessata, i nuclei subiranno un rilassamento e si otterrà un decadimento induttivo libero (FID). Il FID è trasformato di Fourier per dare i picchi dello spettro NMR. Elementi diversi avranno frequenze diverse che porteranno a diversi spostamenti chimici (Tabella 1). Gli spettri NMR possono fornire diversi tipi di informazioni su un composto; l'integrale di un picco fornisce il numero di protoni rappresentati da esso, la costante di accoppiamento (J-coupling) fornisce la correlazione dei protoni e il modello di scissione di un picco dice quanti altri nuclei nmR-attivi sono nelle vicinanze (spesso indicati come "vicini").
Poiché 1H NMR misura 1H nuclei è importante utilizzare solventi deuterati; altrimenti il segnale di interesse andrà perso nel rumore del segnale del solvente.
| Tipo di protone | Maiusc (δ, ppm) | Tipo di carbonio | Maiusc (δ, ppm) |
| 1° Alchil, RCH3 | 0.8–1.2 | 1° Alchil, RCH3 | 0–40 |
| 2° Alchil, R2CH2R | 1.2–1.5 | 2° Alchil, R2CH2R | 10–50 |
| 3° Alchil, RCHR2 | 1.4–1.8 | 3° Alchil, RCHR2 | 15–50 |
| Allylic, R2C=CRCH3 | 1.6–1.9 | Alchene, C=C | 100–170 |
| Chetone, RC(=O)CH3 | 2.1–2.6 | Aryl, C in anello aromatico | 100–170 |
| Etere, ROCH2R | 3.3–3.9 | Alcol o etere, R3COR | 50–90 |
| Alcool, HOCH2R | 3.3–4.0 | Acido carbossilico o estere, RC(=O)OR |
160–185 |
| Vinilico, R2C=CH2 | 4.6–5.0 | Aldeide o chetone, RC(=O)R |
182–215 |
| Vinilico, R2C=CRH | 5.2–5.7 | ||
| Aromatico, ArH | 6.0–8.5 | ||
| Aldeide RC(=O)H | 9.5–10.5 | ||
| Idrossile alcolico, ROH | 0.5–6.0 | ||
| Carbossilico, RC(=O)OH | 10–13 |
Tabella 1. Cambiamenti chimici NMR comuni di protoni e carbonio. 2
Procedura
1. Preparazione del materiale di partenza NMR
- Aggiungere ~ 10 mg di materiale di partenza a un tubo NMR pulito.
- Sciogliere il materiale di partenza in ~0,7 mL di solvente deuterato (esempio dato CDCl3). Un'altezza adatta del solvente per un buon spettro è di 4,5-5 cm.
- Tappare attentamente il tubo NMR e scrivere il nome del campione sul cappuccio.
- Agitare delicatamente il campione per assicurarsi che tutto il materiale si sia sciolto. Fare attenzione ad evitare il contatto tra il solvente e il tappo, che potrebbe portare a possibili contaminazioni del campione.
- Inserire accuratamente il tubo NMR in uno spinner. Lo spinner ruoterà una volta inserito nel magnete per garantire che l'intero campione sperimenti un campo magnetico omogeneo. Pulire l'esterno del tubo NMR e dello spinner con 2-propanolo e tessuti di laboratorio per rimuovere impronte digitali e sporco.
- Posizionare lo spinner in un profondimetro per campioni per assicurarsi che il fondo del tubo NMR non sia inserito troppo lontano nella sonda NMR in quanto ciò potrebbe danneggiare lo spettrometro. Sonde diverse hanno profondità di campionamento diverse e l'utente deve essere a conoscenza del profondimetro specifico.
- Posizionare il campione nello spettrometro NMR. Qui è stato utilizzato uno spettrometro Varian 400 MHz, dotato di un autocampionatore.
- Dopo aver completato la misurazione NMR, elaborare lo spettro e assegnare i picchi nello spettro.
2. Preparazione di 3 M NaOH e sintesi di calcone
- Aggiungere 60 mg di NaOH a un matraccio volumetrico da 50 mL.
- Sciogliere il NaOH aggiungendo acqua deionizzata a metà del pallone. Diluire ulteriormente la soluzione aggiungendo altra acqua fino a raggiungere il segno.
- Aggiungere 10 ml di etanolo a un matraccio a fondo tondo da 50 ml dotato di una barra magnetica.
- Successivamente, aggiungere 680,5 mg di 4-metossibenzaldeide e 5 mL di soluzione di NaOH preparata al punto 2.1 allo stesso matraccio.
- Aggiungere successivamente 671 mg di 4-metilacetofenone alla soluzione agitata e tappare il matraccio e mescolare a temperatura ambiente.
- Monitorare l'avanzamento della reazione mediante 1H NMR a intervalli di 30 minuti (vedere fase 3) fino al pieno consumo di materie prime.
- Aggiungere 5 ml di acqua quando la reazione ha raggiunto il completamento (~3 h). Filtrare il precipitato risultante e lavarlo con 20 ml di etanolo/acqua 1:2. Lasciare asciugare il precipitato all'aria.
- Calcola la resa del prodotto ottenuto. Preparare un campione NMR secondo il punto 1.2.2.7. Controllare la purezza con 1H NMR. Se non puro, purificare il prodotto tramite ricristallizzazione con etanolo.
- Aggiungere circa 3 gocce di miscela di reazione a un tubo NMR utilizzando una pipetta Pasteur e risciacquare la pipetta con solvente deuterato.
- Ripetere i passaggi da 1.2 a 1.8.
3. Breve interpretazione di uno spettro NMR
- Elaborare lo spettro con un programma adatto (esempio dato MestReNova).
- Correlare i diversi picchi agli spostamenti NMR nella Tabella 1. I cambiamenti chimici danno un indizio del tipo di ambiente in cui esistono i protoni.
- Integrare i picchi per dare il numero di idrogeni corrispondenti a ciascun picco. L'integrazione di tutti i picchi dà un numero relativo di protoni totali.
- Valutare la scissione dei picchi di protoni, che indicano il numero di vicini.
- Misurare l'accoppiamento J per vedere come i protoni sono collegati tra loro.
Risultati
Confrontando gli spettri delle materie prime (Figure 1 e 2) con quelli del prodotto finale (Figura 5) si può osservare una chiara differenza tra gli spettri, che indica la formazione del calcone. L'endpoint della reazione può essere determinato prelevando campioni NMR a diversi intervalli di tempo; ad esempio, il picco del protone aldeide (C(=O)H) (1) può essere visto in Figura 3 ma non in Figura 4, a significare il completamento della reazione dopo 3 ore. Osservando gli integrali, i modelli di scissione e gli accoppiamenti J dei picchi, è possibile convalidare la struttura del calcone. Gli integrali dei picchi (numeri sotto il picco, Figura 5)mostrano la quantità relativa di idrogeno presente che dovrebbe essere correlata alla quantità di idrogeno presente nel prodotto. Inoltre, il modello di scissione fornisce un'indicazione del numero di vicini; ad esempio, il picco (5) e (1) - entrambi singlet - indicano che nessun vicino vicino con integrali di 3 è correlato rispettivamente con il gruppo MeO- e Me- . Confrontando gli spostamenti chimici negli spettri con la Tabella 1 è possibile chiarire che il gruppo MeO corrisponde al singoletto a 3,80 ppm e il gruppo Me a 2,45 ppm. Inoltre, la formazione del doppio legame può essere vista come due doppietti (Figura 5), 7,80 e 7,44 ppm. Osservando l'accoppiamento J di 16 Hz si indica la formazione di un E-alchene; l'alchene Zha tipicamente un valore minore di 10-12 Hz.3 L'assegnazione dei picchi aromatici verifica la struttura (Figura 5). 4
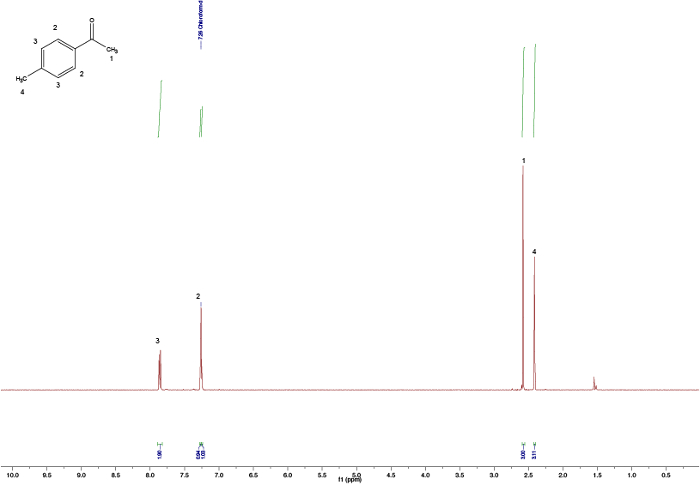
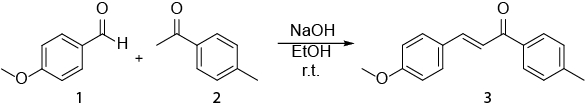
Figura 1. Spettri NMR 1H assegnatidi 4-metilacetofenone Fare clic qui per visualizzare una versione più grande di questa figura.
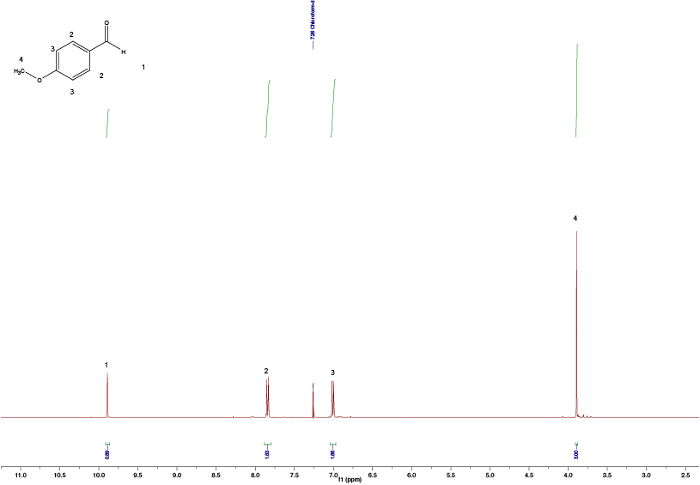
Figura 2. Assegnati spettri NMR 1H di 4-metossibenzaldeide. Fare clic qui per visualizzare una versione più grande di questa figura.

Figura 3. Spettri NMR grezzi da 1 H dopo 30 minuti che mostrano il picco di aldeide residua. Fare clic qui per visualizzare una versione più grande di questa figura.
Figura 4. Spettri NMR grezzi da 1 H dopo 3 ore che non mostrano alcun picco residuo di aldeide. Fare clic qui per visualizzare una versione più grande di questa figura.

Figura 5. SpettriNMR 1H del prodotto ottenuto dopo il work-up. L'immagine inserita mostra gli accoppiamenti a Jdell'alchene. Fare clic qui per visualizzare una versione più grande di questa figura.
Applicazione e Riepilogo
La NMR può, ad esempio, essere utilizzata per rilevare intermedi di reazione, facilitando il lavoro di chiarimento di un meccanismo di reazione. Con l'aiuto della NMR è anche possibile osservare movimenti molecolari e interazioni importanti per lo sviluppo di farmaci. Inoltre, NMR può fornire informazioni strutturali sui materiali solidi; ad esempio per fornire una motivazione per le proprietà osservate dei materiali. Altre applicazioni della NMR possono essere trovate nel campo della medicina, dove la risonanza magnetica (MRI) viene spesso utilizzata per la diagnosi medica. La NMR è stata anche utilizzata in metabolomica per rilevare diversi metaboliti escreti da un organismo, fornendo così un'impronta metabolica. Gli usi della NMR sono ampi; dalla determinazione della struttura di una singola molecola all'imaging del cervello umano.
Riferimenti
- Ta, L., Axelsson, A., Bijl, J., Haukka, M., Sundén, H., Ionic Liquids as Precatalysts in the Highly Stereoselective Conjugate Addition of α,β-Unsaturated Aldehydes to Chalcones. Chem. Eur. J. 20 (43), 13889-13893 (2014).
- Table adapted from Graham Solomons, T. W. Fryhle, C. B., Organic Chemistry, 10th edition, Wiley, p. 387, 418 (2011).
- Clayden, J., Greeves, N., Warren, S., Wothers, P. Proton nuclear magnetic resonance. Organic Chemistry, Chapter 11, Oxford University Press, 269 (2001).
- Wu, X.-F., Neumann, H., Spannenberg, A., Schulz, T., Jiao, H., Beller, M.,Development of a General Palladium-Catalyzed Carbonylative Heck Reaction of Aryl Halides. J. Am. Chem. Soc. 132 (41), 14596-14602 (2010).
Tags
Vai a...
Video da questa raccolta:

Now Playing
Spettroscopia a risonanza magnetica nucleare (NMR)
Organic Chemistry
248.6K Visualizzazioni

Introduzione alla catalisi
Organic Chemistry
34.5K Visualizzazioni

Assemblaggio di un sistema a riflusso per reazioni chimiche riscaldate
Organic Chemistry
168.0K Visualizzazioni

Esecuzione di reazioni al di sotto della temperatura ambiente
Organic Chemistry
70.7K Visualizzazioni

Trasferimento di solventi tramite linea Schlenk
Organic Chemistry
41.7K Visualizzazioni

Degasaggio di liquidi con ciclo freeze-pump-thaw
Organic Chemistry
56.3K Visualizzazioni

Preparazione di reagenti anidri e relativa strumentazione
Organic Chemistry
79.4K Visualizzazioni

Purificazione di composti tramite ricristallizzazione
Organic Chemistry
709.6K Visualizzazioni

Separazione di miscele tramite precipitazione
Organic Chemistry
157.9K Visualizzazioni

Estrazione solido-liquido (lisciviazione)
Organic Chemistry
238.0K Visualizzazioni

Rimozione dei solventi con evaporatore rotante
Organic Chemistry
212.9K Visualizzazioni

Distillazione frazionata
Organic Chemistry
334.7K Visualizzazioni

Preparazione di cristalli per l'analisi mediante diffrazione dei raggi X
Organic Chemistry
32.5K Visualizzazioni

Performing 1D Thin Layer Chromatography
Organic Chemistry
289.9K Visualizzazioni

Cromatografia su colonna
Organic Chemistry
360.6K Visualizzazioni