JoVE 비디오를 활용하시려면 도서관을 통한 기관 구독이 필요합니다. 전체 비디오를 보시려면 로그인하거나 무료 트라이얼을 시작하세요.
Method Article
맞춤형 HPLC 정화 프로토콜 즉, 올리고머 형성 할 수있는 수율 고순도 아밀로이드 베타 42과 아밀로이드 베타 펩티드 (40),
요약
Herein we report a tailored HPLC purification protocol that yields high-purity amyloid beta 42 (Aβ42) and amyloid beta 40 (Aβ40) peptides, capable of oligomer formation. Amyloid beta is a highly aggregation prone, hydrophobic peptide implicated in Alzheimer's disease. The amyloidogenic nature of the peptide makes its purification a challenge.
초록
Amyloidogenic peptides such as the Alzheimer's disease-implicated Amyloid beta (Aβ), can present a significant challenge when trying to obtain high purity material. Here we present a tailored HPLC purification protocol to produce high-purity amyloid beta 42 (Aβ42) and amyloid beta 40 (Aβ40) peptides. We have found that the combination of commercially available hydrophobic poly(styrene/divinylbenzene) stationary phase, polymer laboratory reverse phase - styrenedivinylbenzene (PLRP-S) under high pH conditions, enables the attainment of high purity (>95%) Aβ42 in a single chromatographic run. The purification is highly reproducible and can be amended to both semi-preparative and analytical conditions depending upon the amount of material wished to be purified. The protocol can also be applied to the Aβ40 peptide with identical success and without the need to alter the method.
서문
알츠하이머 병은 신경 퇴행성 질환 효과 만 35 명 이상의 세계이다. 질병의 발병 및 발달에 크게 관여 1은 매우 응집하기 쉬운 소수성 펩타이드 베타 아밀로이드 (Aβ)이다. 이 Aβ가 그러나, 아밀로이드 베타 42 (Aβ42)는 단백질의 가장 독성 형태 42 아미노산 변이체 인 것으로 생각하고, 길이 36 내지 43 개의 아미노산의 범위이다. (3)이 용이 특히 신경 독성 엔티티 것으로 여겨진다 확산 성, 올리고머 성 종을 형성하는 Aβ42의 능력에 대부분 기인한다. 도 4는 Aβ 펩티드에 대한 이해를 증진하기 위하여, 일상적으로 고순도 재료를 얻는 것이 필수적이다. 미량 불순물의 존재는 크게 펩티드 응집 성향의 특성을 변화하는 것으로 나타났다. (5)
티raditionally 같은 소수성 Aβ 펩티드의 고속 액체 크로마토 그래피 (HPLC) 분리 C 사 또는 C 8 실리카 계 정지상과 이동상 산성의 조합을 사용하여 수행되었다. (6) 그러나, 이러한 조건은 펩티드의 정제에 도전을 제공 할 수있다. Aβ 펩티드의 낮은 등전점은 PI (약 5.5) (7)는 산성 조건 하에서, 펩티드의 응집이 증가되고, 그 결과 종종 분리하기 어려운 넓은 비 - 분해 HPLC 피크는 (도 2A)을 생산하는 것을 의미한다. 또한, 이러한 넓은 피크들은 펩티드의 응집 프로필 영향 및 일반적 극적 제조 펩타이드의 양에 영향을 미칠 수있는 정제의 후속 발사가 필요할 수 불순물을 함유한다.
폴리 (스티렌 / 디 비닐 벤젠) 정지상 PLRP-S는을 정제의 대안적인 수단을 나타낸다잉 소수성 펩타이드. 정지상 다른 단백질과 메신저 리보 핵산 (mRNA의) 다수의 정제에 사용되어왔다. 8, 9 PLRP-S 정지상은 역상 분리 추가적인 알킬 리간드를 필요로하지 않고,보다 중요한 펩티드 deaggregation 리드 높은 pH에서 화학적으로 안정하다. 7 여기서, 우리는 고순도 아밀로이드 베타 42 (Aβ42)과 아밀로이드 베타 40 (Aβ40) 펩타이드를 산출 맞춤형 HPLC 정화 프로토콜을보고합니다.
프로토콜
1. Preparative HPLC Purification of the Aβ40 or Aβ42 Peptide
- Prepare the following buffers for the HPLC purification.
- Prepare buffer A (20 mM NH4OH) by adding 1.3 mL of NH4OH (28% solution) to 1,000 mL of ultrapure water.
- Prepare buffer B (80% acetonitrile with 20 mM NH4OH) by adding 1.3 mL of NH4OH (28% solution) to a solution of 800 mL of HPLC-grade acetonitrile and 200 mL of ultrapure water.
- Prepare sample dissolution buffer (0.1% NH4OH) by adding 100 µL of NH4OH (28% solution) to 100 mL of ultrapure water.
- Setup the HPLC instrument as shown in Figure 1.
- Fit the solvent bottles that contain buffer A and buffer B to the inlets of the HPLC pump using polymer tubing. Attach the polymer tubing to the HPLC pump with a one-piece fitting. Ensure that the polymer tubing of each buffer is fitted to the correct inlet valve of the instrument. Fit the HPLC pump with a degasser.
- Couple the HPLC pump with the inlet of the 300 Å 8 µm 25 mm х 300 mm preparative column (Figure 1B, far left column, see Materials List) using polymer tubing.
- Attach the polymer tubing to the preparative column with a one-piece finger tight fitting. Ensure that the polymer column is orientated in the correct manner.
NOTE: The stationary phase of the preparative column is comprised of poly(styrene-divinylbenzene) particles. The correct orientation of the polymer column is marked on the outer casing with a single directional arrow.
- Attach the polymer tubing to the preparative column with a one-piece finger tight fitting. Ensure that the polymer column is orientated in the correct manner.
- Connect the outlet of the column to the dual wavelength detector using polymer tubing and set the wavelength detector to 214 nm and 280 nm.
- Alter the wavelength by changing the detection wavelength parameters in the setup instrument method option of the built-in HPLC software.
- Attach the polymer tubing to the inlet of the wavelength detector with a one-piece finger tight fitting. Attach polymer tubing to the output valve of the wavelength detector. Attach the polymer tubing to the outlet valve of the HPLC detector with a one-piece finger tight fitting. This will be the sample collection tubing.
NOTE: The lack of a strong chromophore on the Aβ peptide dictates that 214 nm be used as the primary ultraviolet (UV) wavelength for peak collection.
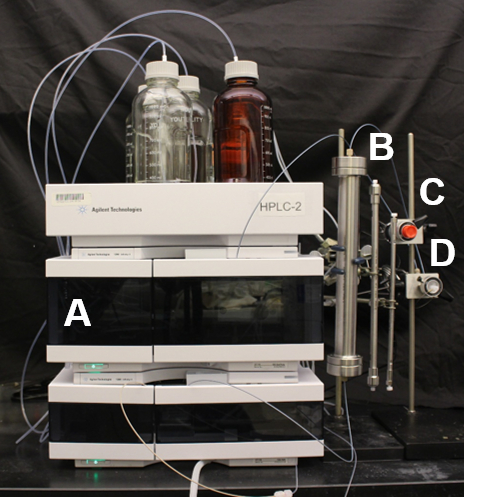
Figure 1: Experimental setup of the HPLC instrument used for purification of the amyloid beta peptides. (A) The quaternary HPLC pump fitted with a degasser and variable wavelength detector set to 214 nm and 280 nm; (B) HPLC columns used for purification of the amyloid beta peptides, from left to right, 25 x 300 mm2 preparative column, 7.5 x 300 mm2 semi preparative column and 4.6 x 250 mm2 analytical column; (C) Manual injector with 20 µL stainless steel injection loop used for analytical HPLC; (D) Manual injector with 10 mL stainless steel injection loop used for preparative and semi preparative purification. Please click here to view a larger version of this figure.
- Program the HPLC software to run the purification method as shown in Table 1. Enter the purification method by changing the solvent timetable parameter (in the setup instrument method option built into the HPLC software). Turn on the HPLC pump by clicking the "on" button on the HPLC software.
NOTE: The pump will begin supplying starting ratio of buffer A and buffer B through the preparative column and the HPLC instrument.- Leave the system for 30 min to fully equilibrate.
| Time / min | % of Buffer Aa | % of Buffer Bb | Flow Rated / mL min-1 |
| 0 | 80 | 20 | 6 |
| 45 | 75.5 | 24.5 | 6 |
| 45.01 | 80 | 20 | 6 |
| 52.01 | 80 | 20 | 6 |
| 52.02 | 73 | 27 | 6 |
| 85 | 73 | 27 | 6 |
| 92 | 5 | 95c | 6 |
Table 1: Timetable for the purification of the Aβ42 and Aβ40 peptides using the 25 × 300 mm polymer column. aBuffer A - H2O with 20 mM NH4OH; b80% MeCN / 20% H2O with 20 mM NH4OH; cRan for 15 min to wash the column prior to the next injection of sample; dIn order to run a flow rate of 6 mL/min on the HPLC instrumentation, the pressure limit needs to be reduced to 200 bar.
- Purification of the Aβ peptide sample
Note: The crude peptide was obtained through automated solid-phase peptide synthesis.10- Dissolve 3 mg of crude Aβ peptide in 4 mL of the sample dissolution buffer. Sonicate the sample for 30-60 s at room temperature and at a frequency of 40 kHz to aid dissolution.
- Inject the entire sample onto the HPLC column using a 5 mL plastic syringe fitted with a 16-gauge stainless steel needle. Run the purification method as outlined in sub-step 1.3.
NOTE: The system enables sample injection to be done through the use of a manual injector fitted with a 10 mL stainless steel injection loop (Figure 1D). The desired Aβ peptide will elute between 72 and 74 min as a sharp resolved peak (Figure 2C). - Collect the sample into a 50 mL conical centrifuge tube. Confirm the identity of the Aβ peak through direct injection mass spectrometry of the collected eluent.11 Store the eluent for up to 12 h at -20 °C.
NOTE: Storage of the solution for periods longer than 12 h is not advised due to the potential for oxidation of the peptide. - Isolate the purified peptide by flash freezing the collected aliquot/aliquots of the Aβ peptide in liquid nitrogen and lyophilize. Perform lyophilization by freeze-drying the sample at a temperature of -60 °C and a pressure of 20 mTorr for a period of 24 h.
- Run the analytical HPLC protocol as outlined below to determine the purity of the Aβ peptide. Store peptides in their lyophilized form at -20 °C for a period of up to 6 months.
2. Analytical HPLC Analysis of the Purified Aβ Protein
- Prepare the HPLC buffers as outlined in sub-section 1.1. of the above protocol.
- Setup the analytical HPLC as per step 1.2.1 and as depicted in Figure 1 with the 4.6 × 250 mm analytical column (Figure 1B, far right column) and the manual injector with 20 µL stainless steel injection loop (Figure 1C) fitted to the instrument.
- Program the HPLC software to run the analytical method as shown in Table 2 following instructions similar to those in step 1.3.
| Time / min | % of Buffer Aa | % of Buffer Bb | Flow Rate / mL min-1 |
| 0 | 95 | 5 | 1 |
| 30 | 50 | 50 | 1 |
Table 2: Timetable for the HPLC purity analysis of the Aβ peptide. aBuffer A - H2O with 20 mM NH4OH; b80% MeCN / 20% H2O with 20 mM NH4OH.
- Purity analysis of the Aβ peptide
- Prepare a 1 mg/mL solution of the purified peptide through dissolution of the peptide in the sample buffer solution.
NOTE: The buffer recipe can be found in sub-section 1.1. Protein concentration is determined by measuring the protein absorption at 280 nm (A280nm).12 The molar extinction coefficient (ε) used to determine concentration is ε = 1,490 dm3 mol-1 cm-1.13 - Inject 20 µL of the 1 mg/mL (222 µM) solution onto the HPLC column and run the analytical method that was setup in step 2.3.
NOTE: The remaining solution not used for analysis can be flash frozen in liquid nitrogen and lyophilized to recover the Aβ peptide. Lyophilization details can be found in sub-section 1.4.4. The Aβ peptide will elute from the analytical column between 16 and 18 min (Figure 2D). Use the built-in integration analysis software that accompanies the HPLC instrument to determine the purity of the Aβ peptide. Purity is determined by integrating each of the individual peaks on the spectrum and calculating peptide-peak percentage area. Typically, a purification of >95% should be ascertained.
- Prepare a 1 mg/mL solution of the purified peptide through dissolution of the peptide in the sample buffer solution.
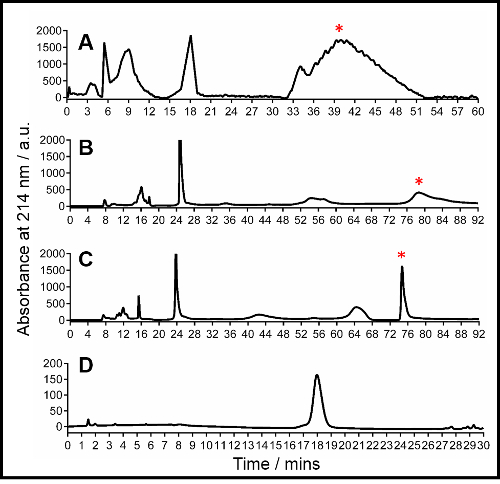
Figure 2: Representative HPLC traces of Aβ42. (A) Traditional C4 silica purification, conditions: Buffer A: H2O with 0.1% trifluoroacetic acid (TFA), buffer B: MeCN (acetonitrile) with 0.1% TFA, gradient: 20 to 27% buffer B over 40 min followed by isocratic 27% buffer B; (B) Preparative purification using the 25 x 300 mm2 polymer column, conditions: Buffer A: H2O with 20 mM NH4OH, buffer B: 80% MeCN / 20% H2O with 20 mM NH4OH, gradient: 20 to 27% buffer B over 70 min followed by isocratic 27% buffer B; (C) Optimized preparative purification using the 25 x 300 mm2 polymer column, conditions are described in Table 1 located in sub-section 1.3 of the protocol description text; (D) Analytical HPLC using the 4.6 x 250 mm2 polymer column, conditions: Buffer A: H2O with 20 mM NH4OH, buffer B: 80% MeCN / 20% H2O with 20 mM NH4OH, gradient-5 to 50% buffer B over 30 min. For parts A, B and C the peak corresponding to Aβ42 is marked by an asterisk. Collection of the marked Aβ42 peak in part C reveals a purity of >95% as shown in part D. Mass spectrometry was used to determine the identity of the Aβ42 peak. Please click here to view a larger version of this figure.
결과
The purification of the Aβ42 peptide using a combination of the PLRP-S stationary phase and a high pH mobile phase results in the formation of a sharp, resolved peak for the Aβ peptide at a retention time between 72 and 74 min (Figure 2C). Confirmation of the identity of the peak is done through direct injection mass spectrometry of the collected eluent. The eluent can be stored at -20 °C in solution for up to 12 h. Longer periods of storage may result in o...
토론
The HPLC purification of the Aβ peptide is highly dependent upon the choice of both the stationary phase employed in the purification and the mobile phase chosen to elute the peptide. The low isoelectric point of the peptide and high propensity for aggregation render traditional chromatographic conditions for the separation of hydrophobic proteins (C4 or C8 stationary phase coupled with an acidic mobile eluent) challenging, with the Aβ peptide eluting as a prolonged broad, non-resolved peak (Figure 2A
공개
The authors have nothing to disclose.
감사의 말
The authors would like to thank Agilent for their technical assistance. Kate Markham and Rafael Palomino are credited for their initial help in the synthesis and purification of the Aβ peptide and Dr Hsiau-Wei Lee is thanked for his help in preparing Figure 1 of the manuscript.
자료
| Name | Company | Catalog Number | Comments |
| Agilent 1260 Infinity II quarternary pump | Agilent | G7111B | http://www.agilent.com/en-us/products/liquid-chromatography/lc-pumps-vacuum-degassers/1260-infinity-ii-quaternary-pump |
| Agilent 1260 Infinity II Dual variable wavelength detector | Agilent | G7114A | http://www.agilent.com/en-us/products/liquid-chromatography/lc-detectors/1260-infinity-ii-variable-wavelength-detector |
| Agilent 1260 Infinity II Manual Injector fitted with 10 mL stainless steel sample loop | Agilent | 0101-1232 | http://www.agilent.com/en-us/products/liquid-chromatography/lc-injection-systems/1260-infinity-ii-manual-injector |
| Agilent 1260 Infinity II Manual Injector fitted with 20 µL stainless steel sample loop | Agilent | G1328C | http://www.agilent.com/en-us/products/liquid-chromatography/lc-injection-systems/1260-infinity-ii-manual-injector |
| Ring Stand Mounting Bracket | Agilent | 1400-3166 | |
| Agilent PLRP-S 300 Å 5 µm 4.6 x 250 mm (Analytical) | Agilent | PL1512-5501 | http://www.agilent.com/en-us/products/liquid-chromatography/lc-columns/biomolecule-separations/plrp-s-for-biomolecules#features |
| Aβ42 or Aβ40 peptide | Synthesized in-house using a CEM liberty automated peptide synthesizer. | ||
| Ammonium Hydroxide (NH4OH, 28% solution) | Fisher Scientific | A669-500 | |
| Acetonitrile | Fisher Scientific | A998-4 | |
| HPLC grade water | Fisher Scientific | W5-4 | |
| Falcon 50 mL conical centrifuge tube | Fisher Scientific | 14-954-49A | |
| Supelco PEEK Fitting One-piece fingertight, pkg of 5 ea | Sigma-Aldrich | Z227250 | |
| Normject 5 cc sterile syringe | Fisher Scientific | 1481729 | |
| 16 Gauge SS Needle | Rheodyne | 3725-086 |
참고문헌
- Querfurth, H. W., LaFerla, F. M. Alzheimer's Disease. N. Engl. J. Med. 362 (4), 329-344 (2010).
- McGowan, E., et al. Aβ42 Is Essential for Parenchymal and Vascular Amyloid Deposition in Mice. Neuron. 47 (2), 191-199 (2005).
- Gong, Y., et al. Alzheimer's disease-affected brain: Presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc. Natl. Acad. Sci. USA. 100 (18), 10417-10422 (2003).
- Selkoe, D. J. Soluble Oligomers of the Amyloid β-Protein Impair Synaptic Plasticity and Behavior. Behav Brain Res. 192 (1), 106-113 (2008).
- Zagorski, M. G., Yang, J., Shao, H., Ma, K., Zeng, H., Hong, A. Methodological and Chemical Factors Affecting Amyloid β Peptide Amyloidogenicity. Methods Enzymol. 309, 189-204 (1999).
- Kim, W., Hecht, M. H. Mutations Enhance the Aggregation Propensity of the Alzheimer's Aβ Peptide. J Mol Bio. 377 (2), 565-574 (2008).
- Fezoui, Y., et al. An improved method of preparing the amyloid beta-protein for fibrillogenesis and neurotoxicity experiments. Amyloid. 7 (3), 166-178 (2000).
- Zhelev, N. Z., Barratt, M. J., Mahadevan, L. C. Use of reversed-phase high-performance liquid chromatography on polystyrene-divinylbenzene columns for the rapid separation and purification of acid-soluble nuclear proteins. J Chromatogr A. 763 (1-2), 65-70 (1997).
- Thess, A., et al. Sequence-engineered mRNA Without Chemical Nucleoside Modifications Enables an Effective Protein Therapy in Large Animals. Mol Ther. 23 (9), 1456-1464 (2015).
- Warner, C. J. A., Dutta, S., Foley, A. R., Raskatov, J. A. Introduction of D-glutamate at a critical residue of Aβ42 stabilizes a pre-fibrillary aggregate with enhanced toxicity. Chem Eur J. 22 (34), 11967-11970 (2016).
- Thompson, J. A., Lim, T. K., Barrow, C. J. On-line High-performance Liquid Chromatography/Mass Spectrometric Investigation of Amyloid-β Peptide Variants Found in Alzheimer's Disease. Rapid Commun. Mass Spectrom. 13 (23), 2348-2351 (1999).
- Layne, E. Spectrophotometric and turbidimetric methods for measuring proteins. Met. Enzymology. 3, 447-455 (1957).
- Ioannou, J. C., Donald, A. M., Tromp, R. H. Characterizing the secondary structure changes occurring in high density systems of BLG dissolved in aqueous pH 3 buffer. Food Hydro. 46, 216-225 (2015).
- Rahimi, F., Maiti, P., Bitan, G. Photo-Induced Cross-Linking of Unmodified Proteins (PICUP) Applied to Amyloidogenic Peptides. J. Vis. Exp. (23), e1071 (2009).
- Bitan, G., Kirkitadze, M. D., Lomakin, A., Vollers, S. S., Benedek, G. B., Teplow, D. B. Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. USA. 100 (1), 330-335 (2003).
재인쇄 및 허가
JoVE'article의 텍스트 или 그림을 다시 사용하시려면 허가 살펴보기
허가 살펴보기This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. 판권 소유