현미경 및 염색: 그램, 캡슐 및 내생포자 염색
Overview
출처: 리안논 M. 레베크1,나탈리아 마틴1,앤드류 J. 반 알스트1,빅터 J. 디리타1
1 미생물학 및 분자 유전학학과, 미시간 주립 대학, 이스트 랜싱, 미시간, 미국
박테리아는 지구상의 거의 모든 곳에서 발견되는 다양한 미생물입니다. 많은 속성은 그램 염색 유형, 모양 및 배열, 캡슐 생산 및 포자의 형성을 포함하되 이에 국한되지 않는 서로 구별하는 데 도움이됩니다. 이러한 특성을 관찰하기 위해, 하나는 빛 현미경 검사를 사용할 수 있습니다; 그러나, 일부 세균성 특성(예: 크기, 착색 부족 및 굴절 특성)은 빛 현미경(1, 2)으로만 박테리아를 구별하기 어렵게 만든다. 세균염색은 가벼운 현미경으로 세균성 모형을 구별할 때 필요합니다. 빛 현미경의 두 가지 주요 유형은 간단하고 화합물입니다. 그 중 가장 큰 차이점은 물체를 확대하는 데 사용되는 렌즈의 수입니다. 간단한 현미경 (예를 들어 확대 유리)에는 물체를 확대하는 렌즈가 하나뿐이지만 복합 현미경에는 배율을 높이기 위해 여러 개의 렌즈가 있습니다 (그림 1). 복합 현미경은 물체의 이미지를 만들기 위해 빛을 수집하는 물체에 가까운 객관적인 렌즈를 가지고 있습니다. 그러면 이미지를 확대하는 안구 렌즈(안구 렌즈)에 의해 확대됩니다. 객관적인 렌즈와 접안렌즈를 결합하면 단일 렌즈만 사용하는 것보다 배율이 높아질 수 있습니다. 전형적으로, 복합 현미경은 다른 배율을 허용하는 다양한 힘의 다중 객관적인 렌즈가 있습니다 (1, 2). 여기에서는 그램 얼룩, 캡슐 얼룩 및 내스포레 얼룩과 박테리아를 시각화하는 것을 논의 할 것입니다.
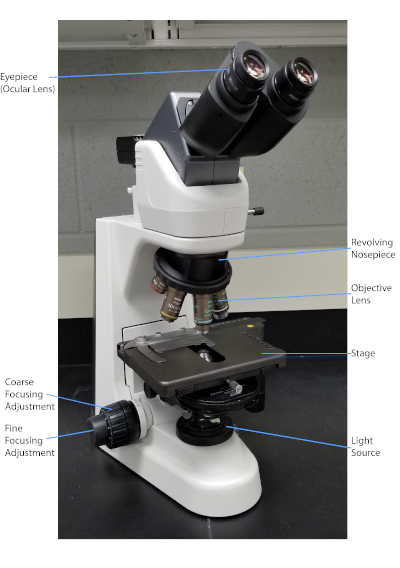
그림 1: 일반적인 복합 현미경. 현미경의 가장 중요한 부분은 표지됩니다.
1884년 덴마크 세균학자 한스 크리스찬 그램(1)이 개발한 그램 얼룩은 세포벽의 조성에 따라 박테리아를 분화한다(1, 2, 3, 4). 간단히 말해서, 세균성 얼룩은 현미경 슬라이드에 배치된 다음 열을 고정하여 세포를 슬라이드에 부착하고 더 쉽게 얼룩 (1)을 받아 들입니다. 열 고정 샘플은 크리스탈 바이올렛으로 염색되어 세포를 보라색으로 바꿔놓았습니다. 슬라이드는 크리스탈 바이올렛을 세포 벽에 고정시키는 요오드 용액으로 플러시되고, 디컬러라이저(알코올)가 들어와 고정되지 않은 크리스탈 바이올렛을 씻어내도록 합니다. 마지막 단계에서, 카운터스테인, 사프란, 색 세포에 빨간색(그림 2)이추가된다. 그람 양성 박테리아 얼룩 보라색 때문에 탈색제에 의해 쉽게 침투되지 않는 두꺼운 펩티도글리칸 층; 그람 음성 박테리아는 더 얇은 펩티도글리칸 층과 더 높은 지질 함량을 가지고 있으며, 탈색제로 탈염되어 사프란이 추가될 때 빨간색으로 대응된다(도 3). 그램 염색은 세포를 두 가지 유형(그램 양성 및 그람 음성)으로 분화하는 데 사용되며 세포 모양(구체 또는 코치, 막대, 곡선 막대 및 나선형) 및 배열(단일 세포, 쌍, 사슬, 그룹 및 클러스터)(1, 3)을 구별하는 데에도 유용합니다.
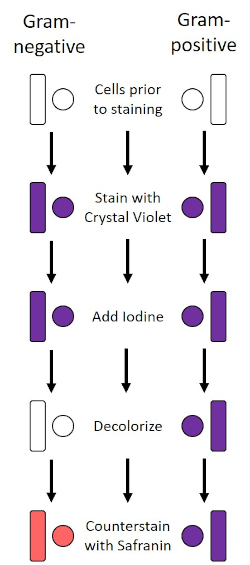
그림 2: 그램 염색 프로토콜의 회로도. 왼쪽 컬럼은 프로토콜의 각 단계에서 그람 음성 박테리아가 어떻게 반응하는지 보여줍니다. 오른쪽 열은 그람 양성 박테리아가 어떻게 반응하는지 보여줍니다. 또한, 도시된 두 가지 전형적인 세균성 세포 모양은 바실리(또는 막대)와 코치(또는 구)이다.
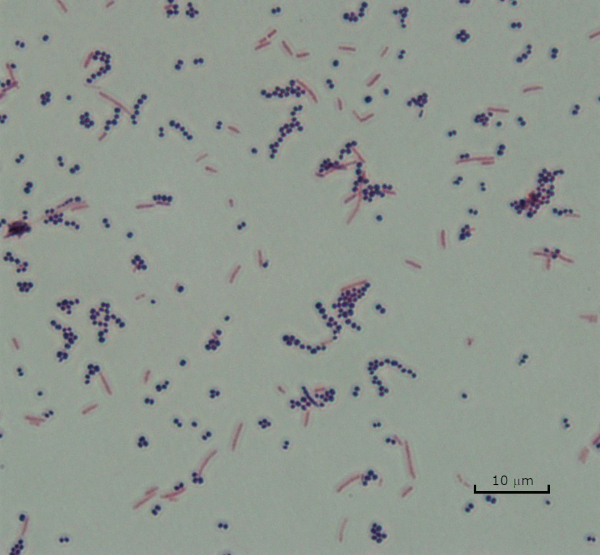
그림 3: 그램 염색 결과. 황색포도상구균(그람 양성 보라색 코치)과 에체리치아 대장균(그램 네거티브 레드 로드)의 혼합물의 그램 얼룩.
일부 박테리아는 캡슐 (3, 5)에게 불린 세포 외점성 외부 층을 생성합니다. 캡슐은 표면 및 기타 박테리아준수, 건조로부터의 보호, 식증으로부터의 보호 등 다양한 기능을 갖춘 보호 구조물입니다. 캡슐은 일반적으로 95% 이상의 물을 함유하는 다당류로 구성되어 있지만 일부는 폴리알코올과 폴리아민(5)을 함유할 수 있다. 그들의 대부분 비 이온 조성 및 얼룩을 격퇴 하는 경향으로 인해, 간단한 염색 방법은 캡슐으로 작동 하지 않습니다;; 대신, 캡슐 염색은 세포와 배경을 얼룩지게 하는 네거티브 염색 기술을 사용하여 캡슐을 세포 주위의 명확한 후광(1, 3) (도 4)로둡니다. 캡슐 염색은 현미경 슬라이드에 산성 얼룩으로 세균성 견본을 얼룩지게 관련시킵니다. 그램 염색과는 달리, 세균성 얼룩은 캡슐 얼룩 동안 열 고정되지 않습니다. 열 고정은 캡슐을 방해하거나 탈수하여 거짓 네거티브 (5)로 이어질 수 있습니다. 더욱이, 열 고정은 캡슐로 착각할 수 있는 세포 의 주위에 청산의 결과로 세포를 수축할 수 있습니다, 거짓 긍정으로 이끌어 내는 (3). 산성 얼룩은 슬라이드 배경을 색; 기본 얼룩, 크리스탈 바이올렛으로 후속 하는 동안, 크리스탈 바이올렛, 박테리아 세포 자체 색상, 캡슐을 염색 하 고 세포와 슬라이드 배경 사이 명확한 후광으로 나타나는 (그림 5). 전통적으로 인도 잉크는 이러한 입자가 캡슐을 관통할 수 없기 때문에 산성 얼룩으로 사용되어 왔습니다. 따라서 캡슐이나 세포는 인도 잉크에 의해 염색되지 않습니다. 대신 배경이 얼룩져 있습니다. 콩고 레드, 니게진, 또는 에오신은 인도 잉크 대신 사용할 수 있습니다. 캡슐 염색은 의사가 환자 샘플에서 배양을 볼 때 세균 감염을 진단하고 적절한 환자 치료를 안내하는 데 도움이 될 수 있습니다. 캡슐화된 박테리아에 기인한 일반적인 질병은 폐염, 뇌막염 및 살모넬라증을 포함합니다.
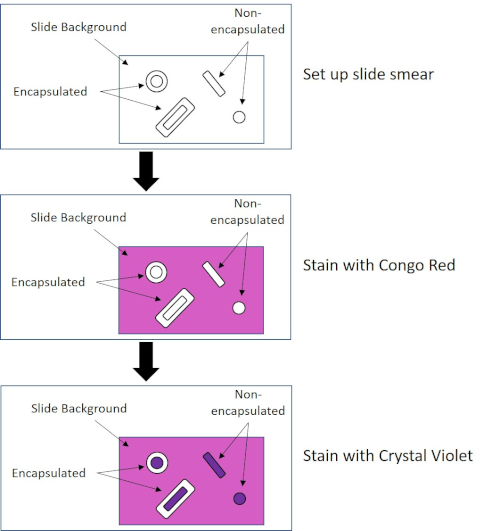
그림 4: 캡슐 염색 프로토콜의 회로도. 상단 패널은 얼룩 적용 전에 슬라이드 얼룩을 표시합니다. 중간 패널은 슬라이드와 박테리아가 기본 얼룩, 콩고 레드를 돌보는 방법을 보여줍니다. 최종 패널은 슬라이드와 박테리아가 카운터스테인 크리스탈 바이올렛을 돌보는 방법을 보여줍니다.
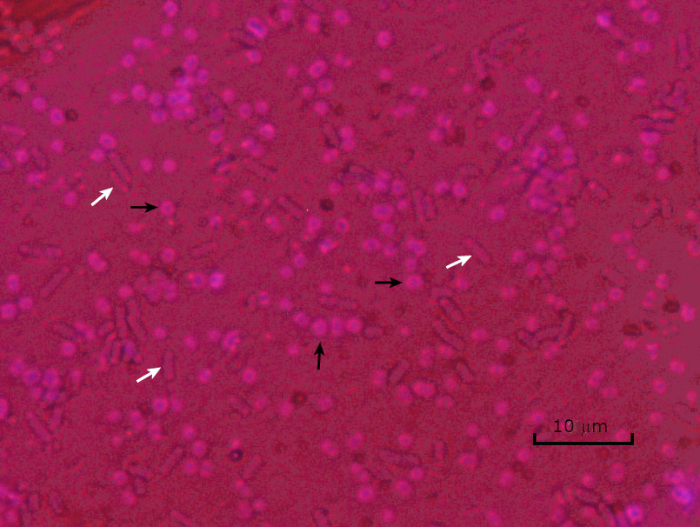
그림 5: 캡슐 염색 결과. 캡슐 에신에토박터 바우마니 (검은 화살로 표시) 및 비 캡슐 에체리치아 대장균 (흰색 화살표로 표시)의 캡슐 염색. 배경이 어둡고 A. 바우마니 세포는 보라색으로 얼룩져 있습니다. A. baumannii 세포 주위의 캡슐은 후광으로 분명하며 대장균에는 후광이 없습니다.
불리한 조건 (예를 들어 영양소 제한, 극한의 온도 또는 탈수)에서 일부 박테리아는 내구포를 생성하며, 신체적 및 화학적 손상에 내성이있는 대사비활성 구조 (1, 2, 8, 9). 내시경 은 박테리아가 세포의 유전 물질을 보호하여 가혹한 조건에서 살아남을 수 있도록; 일단 조건이 성장에 유리한, 포자 발화, 그리고 세균성 성장은 계속합니다. 내시경은 일반적으로 염색에 사용되는 염료에 침투할 수 없기 때문에 표준 염색 기술로 얼룩이 되기 어렵다(1, 9). 내측 포자를 염색하는 데 일상적으로 사용되는 기술은 Schaeffer-Fulton 방법 (도 6)으로,1 차 적인 얼룩 말라카이트 그린을 사용하는, 세포 물질및 열에 상대적으로 약하게 결합하는 수용성 얼룩, 그리고 열, 얼룩이 포자의 피질을 통해 돌파 할 수 있도록 (도 7). 이 단계는 성장하는 세포 (내시경 생물학의 맥락에서 식물 세포라고 불려짐), 내포및 모든 무료 포자 (더 이상 이전 세포 봉투 내에서)를 색을 물고 있습니다. 식물 세포는 말라카이트 그린을 제거하기 위해 물로 세척됩니다. 내포는 포자 내에서 말라카이트 그린을 가열하기 때문에 얼룩을 유지합니다. 마지막으로 식물 세포는 사프란과 함께 비색된다(그림 8). 내시경 포자에 대한 염색은 포자 전자와 비 포자 전자로 박테리아를 분화하는 데 도움이뿐만 아니라, 포자가 샘플에 존재하는지 여부를 결정, 존재하는 경우, 발아시 세균 성 오염으로 이어질 수 있습니다.
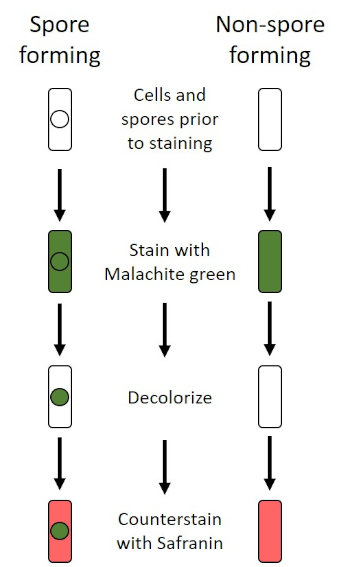
그림 6: 내시경 염색 프로토콜의 회로도. 왼쪽 컬럼은 포자 형성 박테리아가 프로토콜의 각 단계에서 어떻게 반응하는지 보여줍니다. 오른쪽 열은 비 포자 형성 박테리아가 반응하는 방법을 보여줍니다.
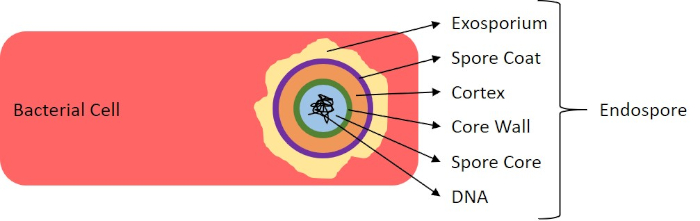
그림 7: 내시경 구조의 다이어그램. 표지된 각종 포자 구조물을 가진 내시경을 포함하는 세균성 세포.
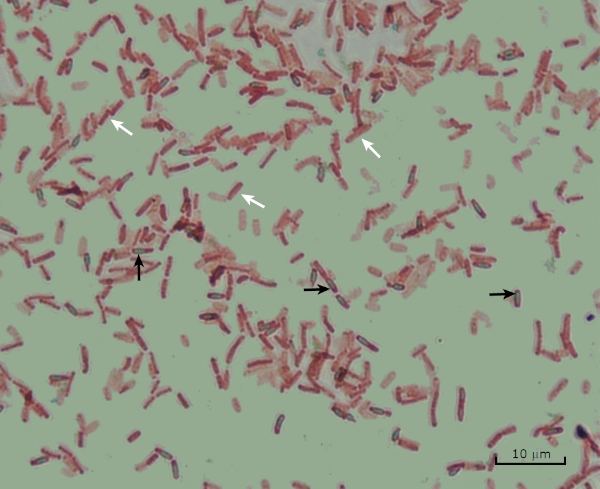
그림 8: 내시경 염색 결과. 바실러스 서브틸리스의내분포의 전형적인 염색. 식물 세포 (흰색 화살표로 표시)는 빨간색으로 얼룩지고 내시경포기 (검은 화살표로 표시)는 녹색으로 얼룩져 있습니다.
Procedure
1화 그램 염색
-
설정
- 염료가 손과 의류를 더럽게 하기 때문에 장갑과 인화성이 없는 실험실 코트를 착용하십시오.
- 분젠 버너는 박테리아를 가열하는 데 사용됩니다. 화염으로 작업할 때주의가 사용됩니다. 긴 머리를 다시 묶습니다.
- 시판되는 그램 얼룩 시약이 사용됩니다.
- 실험실 물티슈로 현미경 슬라이드를 청소하십시오.
-
프로토콜
- 파이프 10 μL 인산염 버퍼링식 식염수 또는 배양 국물을 슬라이드에 밀어.
- 세균성 식민지를 액체에 얼룩져 얇은 짝수 층을 생성합니다.
참고: 너무 오래 된 박테리아는 그램 얼룩 결과 영향을 미칠 것 이다 그들의 세포 벽에 변경 있을 수 있습니다 24 시간 이상 배양을 사용 하지 마십시오 (1, 4). - 완전히 공기 건조 슬라이드.
- 일단 건조되면, 열 은 화염을 통해 슬라이드를 통과하여 박테리아를 수정 (박테리아 측 위로) 4-5 번.
참고: 슬라이드를 화염에 너무 오래 붙이지 않거나 세균 세포(1)를 왜곡할 수 있습니다. - 싱크대 위로 작업, 슬라이드 레벨을 잡고 완전히 열 고정 박테리아를 커버하는 그램의 크리스탈 바이올렛을 적용, 45 초 서 있도록.
- 슬라이드를 비스듬히 잡고 부드럽게 간접적인 물 줄기를 슬라이드에 분출하고 스테인드 박테리아 위로 흘러 내리게 하여 과도한 크리스탈 바이올렛을 헹구십시오. 박테리아에 직접 물을 분출하지 마십시오.
- 슬라이드 레벨을 다시 잡고, 완전히 스테인드 박테리아를 커버하는 그람의 요오드 솔루션을 적용, 45 초 서 있도록.
- 위의 단계 1.2.6에서와 같이 여분의 요오드를 헹구는 다.
- 슬라이드를 비스듬히 유지하면서 슬라이드에 탈색제를 몇 방울 더 추가하여 유출이 명확해질 때까지 얼룩진 박테리아 위로 흘러 내리게합니다. 일반적으로 약 5초. 위의 1.2.6 단계와 같이 물로 즉시 헹구는 다.
참고: 이것은 프로토콜에서 중요한 단계입니다. 탈색제가 너무 오래 또는 충분히 오래 흘러 가도록 하면 거짓 그램 염색(4)이 발생합니다. - 슬라이드 레벨을 다시 잡고, 완전히 박테리아를 커버하는 그램의 사프란을 적용, 45 초 서 있도록.
- 위의 1.2.6 단계에서와 같이 과잉 사프란을 헹두는다.
- 얼룩, 문지르지 말고, 종이 타월을 사용하여 슬라이드에서 물을 과도하게 사용하십시오.
- 100X 목표로 오일 침지로 현미경슬라이드를 검사합니다.
-
결과 및 데이터 분석
- 그램 양성 박테리아는 보라색얼룩것입니다.
- 그람 음성 박테리아는 붉은 얼룩것입니다.
- 박테리아의 모양 (코치, 바실리, 곡선 막대, 나선형)이 표시됩니다.
- 세균 세포의 배열 (단일 세포, 쌍세포, 세포의 사슬, 클러스터, 그룹화)이 볼 수 있습니다.
2. 캡슐 염색
-
설정
- 장갑과 실험실 코트를 염색손과 의류로 착용하십시오.
- 1% 크리스탈 바이올렛 용액을 준비하려면 0.25 그램 크리스탈 바이올렛과 25 mL 증류수를 혼합하여 용해 될 때까지 하십시오.
- 1% 콩고 레드 솔루션을 준비하려면 0.25 그램 콩고 레드와 25 mL 증류수를 혼합하여 용해 될 때까지 하십시오.
- 실험실 와이프와 슬라이드를 청소합니다.
-
프로토콜
- 슬라이드에 10 μL 콩고 레드를 놓습니다.
- 파이펫 팁을 사용하여 세균 성 식민지를 염료에 얼룩져 얇은 짝수 층을 생성합니다.
- 염료/세포 혼합물이 있는 공기 건조 슬라이드는 5-7분입니다.
참고: 가열이 캡슐을 탈수하거나 왜곡 할 수 있기 때문에 가열하지 마십시오. - 1 분 동안 1 % 크리스탈 바이올렛으로 얼룩을 범람.
- 슬라이드를 비스듬히 잡고 부드러운 간접적인 물 줄기를 슬라이드에 분출하고 스테인드 박테리아 위로 흘러 내리게하여 과도한 얼룩을 헹구십시오. 박테리아에 직접 물을 분출하지 마십시오.
- 완전히 공기가 건조 될 때까지 45도 각도로 슬라이드를 유지합니다.
- 100X 목표로 오일 침지 하에서 현미경에 얼룩을 검사합니다.
-
결과 및 데이터 분석
- 세균 세포는 보라색 얼룩 것입니다.
- 슬라이드의 배경은 어둡게 얼룩질 것입니다.
- 캡슐은 어두운 배경에 대한 세포 주위에 명확한 후광이 될 것입니다.
3. 내시경 스테인닝 (쉐퍼 풀턴 방법)
-
설정
- 장갑과 가연성 이연성 실험실 코트를 착용하여 손과 의류를 염료와 불꽃으로부터 보호합니다.
- 분젠 버너는 박테리아를 가열하는 데 사용됩니다. 화염으로 작업할 때주의가 사용됩니다. 긴 머리를 다시 묶습니다.
- 0.5% 말라카이트 그린 용액을 준비하려면 말라카이트 그린 0.125그램과 25mL 증류수를 녹일 때까지 섞으세요.
- 시판 되는 그램의 사프란 시약 솔루션을 사용 하 여.
- 실험실 와이프와 슬라이드를 청소합니다.
-
프로토콜
- 파이프 10 μl 인산염 완충식식염(PBS) 또는 배양 국물을 슬라이드에 밀어.
- 무균 기술을 사용하여 세균 성 식민지를 액체에 얼룩져 얇은 짝수 층을 생성합니다.
참고: 내시경은 일반적으로 젊은 세포에서 형성되지 않으므로 배양은 18 시간에서 36 시간 (9) 사이가 되는 것이 좋습니다. - 완전히 공기 건조 슬라이드.
- 화염을 통해 슬라이드(박테리아 측면 위로)를 4-5번 전달하여 가열합니다.
- 염료를 함유하려면 렌즈 종이 조각(세균 얼룩에 맞게 잘라)을 열 고정 얼룩 위에 놓습니다.
- 말라카이트 그린 용액으로 렌즈 용지를 포화시합니다.
- 뜨거운 접시에 끓는 물 비커 위에 슬라이드를 놓고, 스팀 슬라이드를 5분간 놓고 필요에 따라 한 번에 더 많은 염료를 추가하여 렌즈 종이를 촉촉하게 유지합니다.
참고: 염료 용액이 과열되고 건조되는 것을 피하십시오. - 비커에서 슬라이드를 제거하고 렌즈 용지를 제거하고 폐기하고 슬라이드가 2 분 동안 식힙니다.
- 슬라이드를 비스듬히 잡고, 부드럽고 간접적인 물 줄기를 슬라이드에 분출하여 완전히 헹구어 얼룩 위로 흘러 내리게 합니다.
- 슬라이드 레벨을 잡고, 사프란과 홍수 얼룩, 1 분을 서 있도록.
- 위의 단계 3.2.9에서와 같이 과잉 사프란을 헹노세요.
- 공기 건조를 허용합니다.
- 100X 목표로 오일 침수 하에서 현미경슬라이드를 검사합니다.
-
결과 및 데이터 분석
- 포자는 녹색으로 얼룩질 것입니다.
- 식물 세포는 붉은 얼룩것입니다.
- 일부 식물 세포는 포자를 포함할 것입니다. 세포는 붉은 색으로 얼룩지고 내시경포레스는 녹색으로 얼룩지게됩니다.
Application and Summary
박테리아는 그들의 식별에 도움이 될 수 있는 구별 하는 특성. 이러한 특성 중 일부는 염색 및 가벼운 현미경 검사법에 의해 관찰 될 수있다. 이러한 특성을 관찰하는 데 유용한 세 가지 염색 기술은 그램 염색, 캡슐 염색 및 내시경 염색입니다. 각 기술은 박테리아의 다른 특성을 식별하고 의사가 환자를위한 치료를 권장하고, 샘플 이나 식품의 잠재적 인 오염 물질을 식별하고, 샘플 멸균을 확인하는 데 사용할 수 있습니다.
References
- Black, J. G. Microbiology Principles and Explorations, 4th edition. Prentice-Hall, Inc., Upper Saddle River, New Jersey. (1999)
- Madigan, M. T. and J. M. Martinko. Brock Biology of Microorganisms, 11th edition. Pearson Prentice Hall, Upper Saddle River, New Jersey. (2006).
- Leboffe, M. J., and B. E. Pierce. A Photographic Atlas for the Microbiology Laboratory, 2nd ed. Morton Publishing Company, Englewood, Colorado. (1996).
- Smith, A. C. and M. A. Hussey. Gram stain protocols. Laboratory Protocols. American Society for Microbiology, Washington, DC. Available from: http://www.asmscience.org/content/education/protocol/protocol.2886. (2005).
- Hughes, R. B. and A. C. Smith. Capsule Stain Protocols Laboratory Protocols. American Society for Microbiology, Washington, DC. Available from: http://www.asmscience.org/content/education/protocol/protocol.3041. (2007).
- Anthony, E. E. Jr. A note on capsule staining. Science 73(1890):319-320 (1931).
- Finegold, S. M., W. J. Martin, and E. G. Scott. Bailey and Scott's Diagnostic Microbiology, 5th edition. The C. V. Mosby Company, St. Louis, Missouri. (1978).
- Gerhardt, P., R. G. E. Murray, W. A. Wood, and N. R. Krieg. Methods for general and molecular bacteriology. ASM Press, Washington, DC. (1994).
- Hussey, M. A. and A. Zayaitz. Endospore Stain Protocol. Laboratory Protocols. American Society for Microbiology, Washington, DC. Available from: http://www.asmscience.org/content/education/protocol/protocol.3112. (2007).
Tags
건너뛰기...
이 컬렉션의 비디오:

Now Playing
현미경 및 염색: 그램, 캡슐 및 내생포자 염색
Microbiology
363.5K Views

위노그라드스키 칼럼 생성: 퇴적물 검체에서 미생물 종을 풍부하게하는 방법
Microbiology
129.5K Views

연속 희석 및 플레이팅: 미생물 나열
Microbiology
316.3K Views

농축 배양: 선택 및 차동 매체에서 호기성 및 혐기성 미생물 배양
Microbiology
132.1K Views

순수 배양 및 줄무늬 평판배양: 혼합 검체에서 단일 박테리아 군집 분리
Microbiology
166.2K Views

16S rRNA 시퀀싱: 박테리아 종 식별을위한 PCR 기반 기술
Microbiology
189.1K Views

성장 곡선: 군집 형성 단위 및 광학 밀도 측정을 사용하여 성장 곡선 생성
Microbiology
296.5K Views

항생 물질 감수성 시험: 두 항생제의 MIC 값을 결정하고 항생 시너지를 평가하기 위한 Epsilometer 검사
Microbiology
93.8K Views

플라크 분석: 플라크 형성 단위(PFU)로서 바이러스 역가를 결정하는 방법
Microbiology
186.3K Views

적응 염화칼슘 절차를 이용한 E.coli 세포의 형질변환
Microbiology
86.9K Views

접합: 공여 E.coli에서 수용 E.coli로 암피실린 내성을 전달하는 방법
Microbiology
38.3K Views

파지 형질도입: 공여 E.coli에서 수용 E.coli로 암피실린 내성을 전달하는 방법
Microbiology
29.1K Views
Copyright © 2025 MyJoVE Corporation. 판권 소유