Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Monitoring Equilibrium Changes in RNA Structure by 'Peroxidative' and 'Oxidative' Hydroxyl Radical Footprinting
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
This protocol describes how to quantify the Mg(II)-dependent formation of RNA tertiary structure by two methods of hydroxyl radical footprinting.
Streszczenie
RNA molecules play an essential role in biology. In addition to transmitting genetic information, RNA can fold into unique tertiary structures fulfilling a specific biologic role as regulator, binder or catalyst. Information about tertiary contact formation is essential to understand the function of RNA molecules. Hydroxyl radicals (•OH) are unique probes of the structure of nucleic acids due to their high reactivity and small size.1 When used as a footprinting probe, hydroxyl radicals map the solvent accessible surface of the phosphodiester backbone of DNA1 and RNA2 with as fine as single nucleotide resolution. Hydroxyl radical footprinting can be used to identify the nucleotides within an intermolecular contact surface, e.g. in DNA-protein1 and RNA-protein complexes. Equilibrium3 and kinetic4 transitions can be determined by conducting hydroxyl radical footprinting as a function of a solution variable or time, respectively. A key feature of footprinting is that limited exposure to the probe (e.g., 'single-hit kinetics') results in the uniform sampling of each nucleotide of the polymer.5
In this video article, we use the P4-P6 domain of the Tetrahymena ribozyme to illustrate RNA sample preparation and the determination of a Mg(II)-mediated folding isotherms. We describe the use of the well known hydroxyl radical footprinting protocol that requires H2O2 (we call this the 'peroxidative' protocol) and a valuable, but not widely known, alternative that uses naturally dissolved O2 (we call this the 'oxidative' protocol). An overview of the data reduction, transformation and analysis procedures is presented.
Protokół
1. Preparation of Footprinting Reagents
- Prepare a 10x reaction buffer containing 100 mM sodium cacodylate, 1 mM EDTA, and 1 M KCl. Adjust the pH to 7.4. Filter the buffer using a 0.2 μM acetate filter device (Nalgene). Remark: do not pipet RNA directly into 10x buffer.
- Prepare the titration reaction mix for each reaction as indicated in Table 1. The volume of the titration mix (1x buffer and Mg(II) at the desired concentration) should be 90 μl, before adding 10μl of RNA in 1x buffer.
- Prepare a RNAse T1 digestion buffer containing 6.63M Urea, 20mM sodium citrate, 1mM EDTA, 0.25 μg/μl tRNA, 0.025% xylene cyanol, and 0.025% bromphenol blue. This buffer can be stored at 4 °C for up to 6 months.
- The Fenton reaction requires freshly prepared aqueous solutions of 250 mM Fe(NH4)2(SO4)2, 250 mM EDTA, and 500 mM sodium L-ascorbate. The concentrations noted in chapter 1.5 are optimal for equilibrium experiments in buffer such as sodium cacodylate that does not scavenge radicals and diverse divalent ion concentrations.
- Reagents for the peroxidative (1.5.1) and oxidative (1.5.2) footprinting reaction.
- The peroxidative Fenton reaction requires freshly prepared aqueous solutions of 100 mM Fe-EDTA solution, and 50 mM sodium L-ascorbate and 1.5% H2O2. The Fe-EDTA solution is prepared by mixing Fe(NH4)2(SO4)2 with EDTA to have 1.1 times excess of EDTA over iron.
- Just before initiating the oxidative cleavage reaction (3.4.2), create the Fenton reaction mix by combining 2 μl of Fe(NH4)2(SO4)2 (250 mM), 2.2 μl of EDTA (250 mM), 62.5 μl sodium L-ascorbate (500 mM), 22.5 μl 10x reaction buffer and 135.5 μl of H2O to reach the final concentrations in the footprinting reaction mixture (3.4.2) of 0.10 mM, 0.11 mM, and 6.61 mM, respectively.
2. RNA Preparation for Footprinting Experiment
- RNA can be produced by standard in vitro transcription of DNA templates6 or, if shorter than 50 nucleotides, be purchased (e.g. at Integrated DNA Technologies, www.idtdna.com). Follow recommended laboratory cleanliness procedures to maintain sample integrity of RNA during footprinting experiments.7
- Dephosphorylation8 of in vitro transcribed RNA is necessary prior to 32P labeling of the 5' end of RNA. 10 pmol of RNA are end-labeled by kinasing.9 The solution should be cloudy after successful inactivation of phosphatase.
- Purify radioactively labeled RNA using denaturing gel electrophoresis. The RNA is recovered by two subsequent gel extractions using 0.3 M sodium acetate (pH 5.5). RNA is precipitated by mixing 0.5 ml aqueous solution with 1 ml ethanol and subsequent incubation for 1 minute on dry ice. Spin sample at 4 °C, 12500 rpm for 0.5 hr. Discard the supernatant and wash RNA pellet with 70% ethanol. Remove supernatant after additional centrifugation and dry pellet in vacuum.
- Dissolve the 32P-labeled RNA samples in 330 ul of 1x Buffer.Pipet 30 μl of the RNA solution in a reaction tube, precipitate with ethanol and dry RNA in vacuum; keep this tube for generating the reference ladder (step 3.5). Denature the buffered RNA solution by heating at 95 °C for 2 min. Cool the samples to room temperature for 15 min and give a quick spin to bring down the condensates on the lids of the tubes back into solution.
3. The Footprinting Experiment
- The electrophoresis comb determines the maximum number of data points per analytical gel. We use a 30 well comb. The reference ladder occupies 2 wells and the uncleaved control one. Prepare 27 reaction tubes. The final Mg(II) concentrations should be evenly spaced on a logarithmic scale spanning orders of magnitude around the titration midpoint.
- Separately, incubate the RNA and the Mg(II) solutions at 50°C for 5 min. Mix 10 μl of RNA solution with 90 μl of the corresponding amount of Mg(II) in 1x buffer to reach a final volume of 100 μl. Incubate the solutions for 30 min at 50°C.
- Let the solutions equilibrate at 25°C for 1 hr while folding occurs.
- Peroxidative(3.4.1) or oxidative (3.4.2) hydroxyl radical footprinting reaction.
- Start the peroxidative reaction by placing droplets of 2 μl Fe-EDTA (100 mM), 2 μl sodium L-ascorbate (50 mM) and 2 μl H2O2 (1.5%) separated from each other at the top inside of the reaction tube containing the RNA solution and start the footprinting reaction by vigorous mixing. Stop the reaction after 15 seconds by adding 300 μl cold absolute ethanol. Turn the tubes 3-5 times. Precipitate, wash and dry the pellet as in step 2.4.
- Start the oxidative hydroxyl radical footprintingreaction by adding 5 μl of the freshly prepared Fenton reaction mix (1.6). Incubate for 30 min at 25°C. Add 300 μl of cold absolute ethanol to quench the reaction and mix by turning the tubes 3-5 times. Precipitate, wash and dry the pellet as indicated in step 2.4.
- Generate a RNase T1 digest reference sample according to standard procedures10 using the solution prepared in step 1.3.
- Dissolve RNA pellets in 8 μl gel loading dye II (AMBION) and confirm RNA is resuspended by using the Geiger counter.
- Prepare a denaturing, 8% polyacrylamide sequencing gel according to standard protocols.11 Load samples including two references and a control. Separate RNA fragments at 60 W - 75 W for 2.5 hr. Expose dried gel to a storage phosphor screen overnight. Scan phosphor screen with an imaging system for filmless autoradiography, e.g. Storm 865 (GE Healthcare) or Typhoon (GE Healthcare). Transfer gel image file to your computer.
4. Data Analysis
- The hydroxyl radical reactivity of each nucleotide is determined by quantifying the intensity of each band on the analytical gel. Download, install, and open SAFA, an easy to use open source software for single band fitting and quantification.12, 13 Follow the user manual instructions. Briefly, load the RNA sequence as .txt file followed by the gel picture as .gel file. Adjust band intensities, define the lanes, choose an anchor lane, and perform a gel alignment. Assignment of bands to nucleotides occurs in reference to RNase T1 digestion ladder. Quantify the band integrals and use the normalization/colorplot feature to normalize and assign potentially invariant residues. Save the output as a .txt file.
- SAFA outputs a spreadsheet containing columns representing lanes on the gel and rows representing the integrated band density of the individual bands corresponding to the RNA fragment. First, identify the protection sites which display a noticeable change in solvent accessibility by comparing the protection profile derived from the no Mg(II) sample with the profile of the end point Mg(II) concentration (50 mM in our example). The lower the value, the more protected the nucleotide is against the attack from hydroxyl radicals and vice versa.
- Create folding isotherms from the normalized band integrals of individual or groups of nucleotides versus Mg(II) concentration. The isotherms are individually scaled to fractional saturation by fi = L + (U - L)•
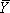 where f denotes the integrated density of the band(s) being analyzed, L and U represent the lower and upper limits to the transition and
where f denotes the integrated density of the band(s) being analyzed, L and U represent the lower and upper limits to the transition and 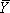 is fractional saturation.14 The data are fit using a non-linear least square analysis program (We use either Origin (OriginLab) or GraphPad (GraphPad Software, Inc.).) to the Hill equation (1),
is fractional saturation.14 The data are fit using a non-linear least square analysis program (We use either Origin (OriginLab) or GraphPad (GraphPad Software, Inc.).) to the Hill equation (1),
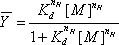
where Kd is the equilibrium dissociation constant, [M] corresponds to the concentration of the variable that mediates the folding reaction, Mg(II) in this example, and nH is the Hill coefficient. This procedure scales the transition to fractional saturation, determines its midpoint and provides a phenomenological test of whether a transition is sigmoidal. Isotherms derived from sequencing gels (Figure 3A) that were analyzed by SAFA (Figure 3B) and fit as described above are shown in Figure 3C. The fractional saturation isotherms shown in Figure 3 were generated by scaling in a spreadsheet the values generated by SAFA against the best fit upper and lower limits (U and L) using the equation fraction = value /(U - L) - L /(U - L).
5. Representative Results:
Figure 3 shows representative results from P4-P6 RNA •OH footprinting experiments. The gel image (left) indicates that a) background cleavage is minimal (right lane), b) single nucleotide assignment is possible due to well defined bands in the T1 lane (RNase T1 cleaves at each G), and c) the hydroxyl radical induced fragmentation of RNA is well above background. The transition from low to high Mg(II) is affiliated with decreasing integrated band density of individual and groups of bands indicating formation of RNA tertiary structure. Single or groups of contiguous nucleotide whose cleavage changes concomitantly are referred to as a 'protection'. The •OH protections characteristic of the Mg(II) mediated folding of the P4-P6 RNA closely correspond to the solvent inaccessible regions of the molecule observed in its crystal structure.15
Folded RNA molecules can have very different extents (i.e., how close to background the band densities decline) of tertiary contact protection that have not been correlated with clear structural or dynamic phenomenon. Thus, some RNA molecules will show well defined structural transitions, as shown in Fig 3, and some will not. Band intensities are quantified and normalized by SAFA12 analysis (Figure 3B). The output is a 'thermal' plot visualizing the relative degree of protection against •OH. The color transition describes the accessibility change upon Mg(II) addition. White to red or blue shows more accessible or more protected nucleotides, respectively. Each degree of shading is affiliated with a numerical value which can be plotted as a protection curve (Figure 3C) and analyzed by a binding model such as the Hill equation (1). The equilibrium dissociation constant affiliated with Protection 153-155 is roughly twice that of the corresponding value of Protection 163-164.
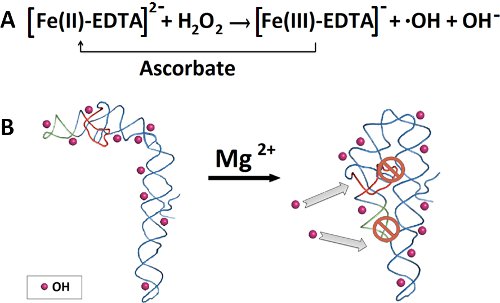
Figure 1. Hydroxyl radical production and P4-P6 RNA folding. A) Fe(II) catalyzes the generation of hydroxyl radicals from hydrogen peroxide. Ascorbate reduces Fe(III) back to ferrous iron. B) In the absence of Mg(II), no P4-P6 tertiary structure is formed, allowing hydroxyl radicals to penetrate and cleave all accessible backbone positions. Addition of the Mg(II) initiates folding of P4-P6, allowing only the solvent accessible backbone to be cleaved by the hydroxyl radicals.
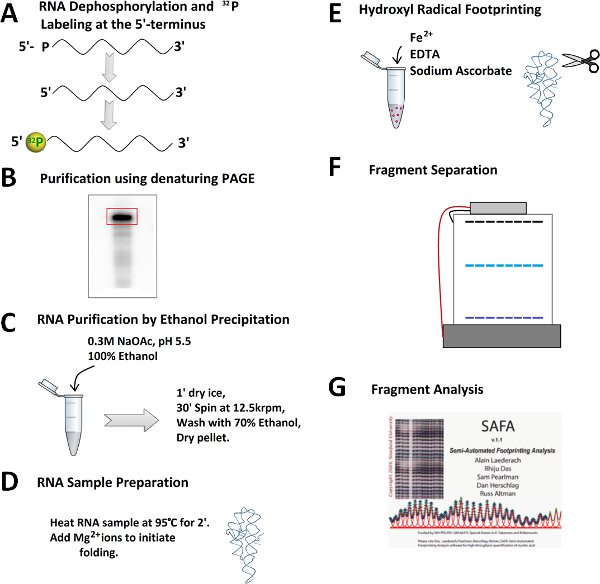
Figure 2. Outline for a hydroxyl radical footprinting experiment. A) Dephophorylation and 32P 5'-end labeling of RNA. B) Purification of 32P-labeled RNA on a denaturing polyacrylamide gel. C) Excision of RNA band, subsequent RNA extraction, and ethanol precipitation. D) Prefolding and folding of RNA. E) Addition of freshly-prepared Fenton reaction mixture to generate the hydroxyl radicals. F) RNA fragment separation by denaturing polyacrylamide gel electrophoresis. G) Quantitation of RNA fragments by SAFA software.
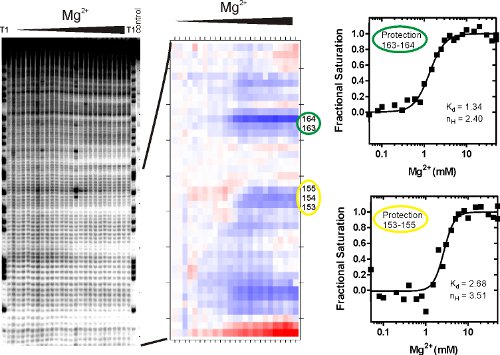
Figure 3. Analysis of RNA fragments after peroxidative Fenton footprinting reaction. (A) The RNA was exposed to hydroxyl radicals and the cleavage products were resolved using denaturing polyacrylamide gel electrophoresis (PAGE). The picture shows peroxidative cleavage products with increasing concentration of Mg(II). Reference and control lanes are labeled. This image file is the input for the SAFA program which quantitates the volume of each band. (B) The 'thermal' plot generated by SAFA. (C) A spreadsheet of values relating integrated band density to nucleotide number output from SAFA is analyzed by a binding model such as the Hill equation (1). Regions of protection were chosen according to the increase in band integral density as a function of Mg(II) concentration. The Kd is the Mg(II)concentration at which half of the RNA is folded at the site being monitored. The Hill coefficient, nH, is a measure of the slope of the curve which gives information on the binding cooperativity and provides a lower estimate for the number of magnesium ions involved in folding of this particular site.
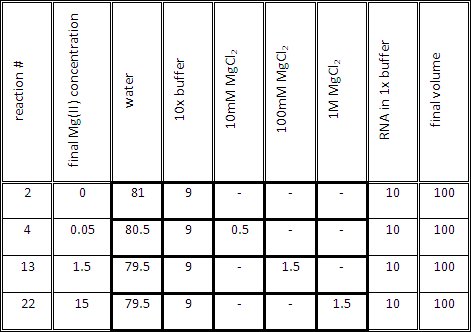
Table 1. Representative volumes for generating RNA samples containing different Mg(II) concentrations.
Dyskusje
Hydroxyl radical footprinting is a valuable tool to assess the solvent accessible surface area of nucleic acids. Qualitative and quantitative formation of tertiary structure14 can be followed as a function of parameters such as ion type and concentration, pH, temperature, binding proteins or folding co-factors. The compelling combination of a straight forward and inexpensive protocol and the resulting solvent accessibility and folding information on a single-nucleotide level makes this method very attractive. ...
Ujawnienia
No conflicts of interest declared.
Podziękowania
This work was supported by grants from the National Institute of Health RO1-GM085130 and National Science Foundation MCB0929394. We thank Dr. Marion Schmidt for her hospitality and for allowing us to film in her laboratory.
Materiały
| Name | Company | Catalog Number | Comments |
| Name | Company | Cat# | |
| Sodium Cacodylate(Caution! Toxic) | Sigma-Aldrich | C4945-25g | |
| EDTA (0.5 M) | Ambion | AM9260G | |
| DEPC treated water | Ambion | AM9915G | |
| Sodium Acetate (3 M) | Ambion | AM9740 | |
| MgCl2 (1 M) | Ambion | AM9530G | |
| Urea | Ambion | AM9902 | |
| Sodium Citrate | Sigma-Aldrich | W302600 | |
| tRNA | Sigma-Aldrich | R-7876 | |
| Sodium-L-ascorbate | Sigma-Aldrich | A7631-25g | |
| Fe(NH4)2(SO4)2 . 6 H2O | Sigma-Aldrich | F1543-500g | |
| RNase T1 | Fermentas | EN0541 | |
| Hydrogen Peroxide (30%) | Sigma-Aldrich | 349887 |
Odniesienia
- Tullius, T. D., Dombroski, B. A. Hydroxyl radical "footprinting": high-resolution information about DNA-protein contacts and application to lambda repressor and Cro protein. Proc. Natl. Acad. Sci. U.S.A. 83, 5469-5473 (1986).
- Celander, D. W., Cech, T. R. Iron(II)-ethylenediaminetetraacetic acid catalyzed cleavage of RNA and DNA oligonucleotides: similar reactivity toward single- and double-stranded forms. Biochemistry. 29, 1355-1361 (1990).
- Celander, D. W., Cech, T. R. Visualizing the higher order folding of a catalytic RNA molecule. Science. 251, 401-407 (1991).
- Sclavi, B., Sullivan, M., Chance, M. R., Brenowitz, M., Woodson, S. A. RNA folding at millisecond intervals by synchrotron hydroxyl radical footprinting. Science. 279, 1940-1943 (1998).
- Tullius, T. D., Dombroski, B. A., Churchill, M., Kam, L. Hydroxyl radical footprinting: a high-resolution method for mapping protein-DNA contacts. Methods. Enzym. 155, 537-558 (1987).
- Milligan, J. F., Groebe, D. R., Witherell, G. W., Uhlenbeck, O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic. Acids. Res. 15, 8783-8798 (1987).
- Schlatterer, J. C., Brenowitz, M. Complementing global measures of RNA folding with local reports of backbone solvent accessibility by time resolved hydroxyl radical footprinting. Methods. 49, 142-147 (2009).
- Sambrook, J., Russell, D. W. . Molecular Cloning: A Laboratory Manual. , (2001).
- Zaug, A. J., Grosshans, C. A., Cech, T. R. Sequence-specific endoribonuclease activity of the Tetrahymena ribozyme: enhanced cleavage of certain oligonucleotide substrates that form mismatched ribozyme-substrate complexes. Biochemistry. 27, 8924-8931 (1988).
- Knapp, G. Enzymatic approaches to probing of RNA secondary and tertiary structure. Methods. Enzym. 180, 192-212 (1989).
- Slatko, B. E., Albright, L. M. Denaturing gel electrophoresis for sequencing. Curr. Protoc. Mol. Biol. Chapter 7, Unit 7.6-Unit 7.6 (2001).
- Das, R., Laederach, A., Pearlman, S. M., Herschlag, D., Altman, R. B. SAFA: semi-automated footprinting analysis software for high-throughput quantification of nucleic acid footprinting experiments. RNA. 11, 344-354 (2005).
- Simmons, K., Martin, J. S., Shcherbakova, I., Laederach, A. Rapid quantification and analysis of kinetic *OH radical footprinting data using SAFA. Methods. Enzym. 468, 47-66 (2009).
- Uchida, T., He, Q., Ralston, C. Y., Brenowitz, M., Chance, M. R. Linkage of monovalent and divalent ion binding in the folding of the P4-P6 domain of the Tetrahymena ribozyme. Biochemistry. 41, 5799-5806 (2002).
- Cate, J. H., Gooding, A. R., Podell, E., Zhou, K., Golden, B. L., Kundrot, C. E., Cech, T. R., Doudna, J. A. Crystal structure of a group I ribozyme domain: principles of RNA packing. Science. 273, 1678-1685 (1996).
- Petri, V., Brenowitz, M. Quantitative nucleic acids footprinting: thermodynamic and kinetic approaches. Curr. Opin. Biotechnol. 8, 36-44 (1997).
- Takamoto, K., Das, R., He, Q., Doniach, S., Brenowitz, M., Herschlag, D., Chance, M. R. Principles of RNA compaction: insights from the equilibrium folding pathway of the P4-P6 RNA domain in monovalent cations. J. Mol. Biol. 343, 1195-1206 (2004).
- Brenowitz, M., Senear, D. F., Shea, M. A., Ackers, G. K. Quantitative DNase footprint titration: a method for studying protein-DNA interactions. Methods. Enzym. 130, 132-181 (1986).
- Shcherbakova, I., Mitra, S. Hydroxyl-radical footprinting to probe equilibrium changes in RNA tertiary structure. Methods. Enzym. 468, 31-46 (2009).
- Howlett, G. J., Minton, A. P., Rivas, G. Analytical ultracentrifugation for the study of protein association and assembly. Curr. Opinion. Chem. Biol. 10, 430-436 (2006).
- McGinnis, J. L., Duncan, C. D., Weeks, K. M. High-throughput SHAPE and hydroxyl radical analysis of RNA structure and ribonucleoprotein assembly. Methods. Enzym. 468, 67-89 (2009).
- Jonikas, M. A., Radmer, R. J., Laederach, A., Das, R., Pearlman, S., Herschlag, D., Altman, R. B. Coarse-grained modeling of large RNA molecules with knowledge-based potentials and structural filters. RNA. 15, 189-199 (2009).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone