Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Viability of Bioprinted Cellular Constructs Using a Three Dispenser Cartesian Printer
W tym Artykule
Podsumowanie
A Cartesian bioprinter was designed and fabricated to allow multi-material deposition in precise, reproducible geometries, while also allowing control of environmental factors. Utilizing the three-dimensional bioprinter, complex and viable constructs may be printed and easily reproduced.
Streszczenie
Tissue engineering has centralized its focus on the construction of replacements for non-functional or damaged tissue. The utilization of three-dimensional bioprinting in tissue engineering has generated new methods for the printing of cells and matrix to fabricate biomimetic tissue constructs. The solid freeform fabrication (SFF) method developed for three-dimensional bioprinting uses an additive manufacturing approach by depositing droplets of cells and hydrogels in a layer-by-layer fashion. Bioprinting fabrication is dependent on the specific placement of biological materials into three-dimensional architectures, and the printed constructs should closely mimic the complex organization of cells and extracellular matrices in native tissue. This paper highlights the use of the Palmetto Printer, a Cartesian bioprinter, as well as the process of producing spatially organized, viable constructs while simultaneously allowing control of environmental factors. This methodology utilizes computer-aided design and computer-aided manufacturing to produce these specific and complex geometries. Finally, this approach allows for the reproducible production of fabricated constructs optimized by controllable printing parameters.
Wprowadzenie
Tissue engineering uses the principles of biology and engineering in the development of functional substitutes to maintain, restore, or enhance native tissue and . The capability of generating three-dimensional biomimetic constructs on demand would facilitate scientific and technological advances in tissue engineering as well as in cell-based sensors, drug/toxicity screening, tissue or tumor models, and other . The three-dimensional organization of tissue-engineered constructs is a fundamental component of the fabrication method because it must closely mimic the highly organized interaction of cells and extracellular matrix in native tissue.
Biodegradable and shape-forming three-dimensional scaffolds are critical factors in generating novel tissue constructs because cells migrate to form a two-dimensional layer of cells, but lack the ability to grow in favored three-dimensional . The scaffold serves as a temporary foundation for cell attachment and proliferation, so it must be constructed from materials with controllable porosity and biodegradability, and sufficient mechanical integrit. The scaffold materials should not be cytotoxic or create an adverse response from the host. Hydrogels have been commonly used in tissue engineering techniques, and due to their hydrophilicity, hydrogels permit fluid and gas exchange throughout the structur. By combining different hydrogels, the synthesized hydrogel’s properties are modifiable to meet distinct application requirement.
The conventional tissue engineering approach involves the creation of acellular porous sacrificial scaffolds that are seeded with cells post-fabricatio. Many techniques have been employed, such as fiber bonding, solvent casting, and melt molding, but proved to be minimally successful for tissue engineering applications. Fiber bonding methods allow fibers to be aligned in specific shapes, but they are only capable of producing very thin scaffold. Solvent casting methods produced highly porous constructs, however the largest produced membrane was only 3-mm thic. Therefore, creating three-dimensional constructs is not feasible using these techniques. Melt molding techniques proved successful in producing three-dimensional scaffolds, but such high temperatures are required that biological materials cannot be incorporated during the production proces. Scaffolds seeded post-fabrication are limited in their ability to meet the requirements of tissue engineering to produce three-dimensional scaffolds with pre-defined or controllable microstructures and . Another major issue with solid scaffold seeding technologies is the deficiency of vascularization and poor mechanical .
Bioprinting has since been extended to three dimensions through the use of nontoxic, biodegradable, thermo-reversible gels to overcome the disadvantages of conventional . A few of the solid freeform fabrication techniques currently being employed are laser-assisted bioprinting and inkjet printing. Laser-assisted bioprinting techniques use a pulsed laser source, a target plate, and a receiving substrate to generate three-dimensional . However, this technique is limited due to low throughput, low cell viability, and can only produce limited arrangements of fabricated structures because only photocrosslinkable prepolymers can be used to form a crosslinked hydrogel . Inkjet printing was developed as a non-contact methodology that reproduces digital image data on a substrate by depositing picoliter ink . However, inkjet printing does not produce a high-resolution construct, constructs experience rapid protein denaturation, and many of the cells are lysed during the deposition .
Currently, new additive manufacturing bioprinting methods have been developed. In these systems cells, proteins, growth factors, and biomimetic hydrogels are typically integrated into matrix materials during the fabrication process and concurrently deposited using computer-controlled actuators to generate three-dimensional scaffold-based cell-laden constructs that closely mimic the microarchitecture of native . The cell-laden hydrogels constitute the bioink, which can be heterogeneous, consisting of multiple cell types, or homogeneous. Additive manufacturing systems deposit bioink drop-by-drop or layer-by-layer via disposable syringes and tips onto a computer-controlled stage capable of moving in the x, y, and z directions. Through computer software, the architecture of printed scaffolds can be easily manipulated depending on requirements of the application. Unlike conventional techniques, three-dimensional medical technologies (magnetic resonance imaging, computer tomography) can be incorporated into the designs, generating patient-specific construct. These methods also allow the possibility of producing vascularized replacements because constructs are produced with a higher local cell density, allowing cell-cell interactions and improving the likelihood of post-implantation surviva.
The Palmetto Printer is a custom built three-dimensional multi-dispenser system that uses programmable robotic manufacturing methods to generate three-dimensional heterogeneous tissue constructs (Figure 1). It allows the use of a plurality of materials in unique combinations to produce heterogeneous structures. The initialization of the bioprinter is one of the most important steps in bioprinting because it allows you to set a variety of parameters to optimize the printability of the bioprinted constructs.
The bioprinter comprises a batch type process with startup, operation and shutdown sequences controlled by a programmable logic controller (PLC), which the user operates through an interactive touch screen control panel (Figure 1, A). To prevent contamination of biological materials the bioprinter is enclosed in a positively-pressured poly(methyl methacrylate) (PMMA) chamber with a high-efficiency particulate arrestance (HEPA)-filtered air circulation system (Figure 1, B,C). The interior of the printer can be sterilized using the built-in ultraviolet light sources (Figure 1, D). The central component of the bioprinter is a fully programmable positioning robot that can reproducibly place a dispenser tip with an accuracy of 10 micrometers (Figure 1, E). There are three dispensers, which are able to deposit volumes as small as 230 nl using a rotary screw (Figure 1, F). They are independently programmable using separate computers that govern printing parameters for each dispenser (Figure 1, G). Rotary-screw dispensing utilizes the rotation of a motor-driven screw to move bioink down a syringe and out of the syringe tip. These dispensers are mounted onto a pneumatically controlled Tool Nest (Figure 2A, B), allowing the robot to switch dispenser mounted onto the Z-axis robotic arm under programmed control (Figure 1, H).
The XYZ robot receives printing instructions from a computer running design software (Figure 1, I). Each program contains dispensing locations, calibration routines, and dispenser-changing protocols. The design of generated constructs primarily consists of the XYZ coordinates where each dispenser will deposit material. The bioprinter comprises two optical light sensors (Figure 2C) that determine the XYZ coordinates of the syringe tip end. These sensors send coordinate information to the robot, which uses these to calculate positions of the dispenser tip ends. There is an additional displacement laser (Figure 2D) that projects a 633 nm diode red laser beam of spot size 30 x 100 micrometers to measure distance with an accuracy of 0.1 micrometers. When the beam is highly focused the robot determines the Z distance of the printing surface. This measurement, and the optical light sensors measurement of the tip end in Z, allows calculation of accurate Z coordinates used to place the dispenser tip in relation to the printing surface. The dispenser tips move laterally and vertically through the X-axis oriented optical light sensor to find the Y and Z centers, and laterally through a Y-axis sensor to find the center of the X-axis. The printing surface is mapped using the formula for a flat plane in xyz space: ax + by +cz = d to determine where the surface is relative to the position of the dispensing tip end. The printer stage (Figure 1, J) holds a sample Petri dish up to 80 mm in diameter and uses a recirculating water bath to maintain the set temperature (Figure 1, K). Stage temperature can be set within a range of -20 and remains stable within . There is a USB camera mounted onto the robot Z-arm to provide a magnified view of the dispensing tip during the printing process (Figure 1, L). There is a second camera mounted towards the top of the chamber interior that provides a complete view of the bioprinter during the printing process (Figure 1, L).
A computer-aided design drawing software determines the deposition pattern and permits the user to generate incrementally spaced droplets and complex structures (Figure 3). Three-dimensional pathways can be manually coded into the printer-compatible design software or imported from a separate computer-aided design drawing software (Figure 4, Table 1). The printer-compatible software allows variations of printing parameters such as the deposition method (single droplet deposition or continuous pathway deposition), three-dimensional geometry of the pathways, deposition rate, distance between the syringe tip end and substrate printing surface, the amount of time to deposit an individual drop, and the height and speed the syringe is lifted between deposition of the drops. Each program contains XYZ dispensing locations, tip calibration routines, and dispenser-changing protocols to provide a sterile environment, without operator intervention, during printing. The programmable logic controller (PLC) of the robot receives instructions from the computer running the design software and controls the timing of events from the external controllers (e.g., the dispensers). To do this, the PLC uses a looping mechanism to control the dispensers, robotic positioning device, and environmental factors.
Three-dimensional direct-write bioprinting utilizing a rotary-screw, liquid-dispensing system allows the process of depositing cells to be more efficient, accurate, and easier than previous methods. This study shows the custom built bioprinter is capable of generating cell-laden hydrogel constructs with high cell viability.
Protokół
1. Preparation of Gelatin Containing Substrate for Three-Dimensional Bioprinting of Alginate Hydrogels
- Prepare the calcium/gelatin substrate following the calcium/gelatin substrate method described by Pataky et al11 to avoid reduced viability associated with high content. The calcium/gelatin substrate method is listed below.
- Combine calcium chloride dehydrate (1.5 wt%), sodium chloride (0.9 wt%), and porcine gelatin (2 wt%) in distilled water and boil for 2 min to create a 100 mM gelatin solution.
- Pour 5 ml of the gelatin/calcium solution into 100 mm standard petri dishes, swirl the solution around to make an even coating on the surface, and place on a flat surface in the fridge to gel O/N (allow to gel at least 8 hr before use).
- To increase the opacity of the substrate surface, add titanium dioxide (0.3 wt%) to the gelatin/CaCl2 solution. Stir for 10 min. Autoclave the gelatin/TiO2 solution on the liquids cycle for 30 min to sterilize it.
- Add 3 ml of the gelatin/TiO2 solution to the surface of the previously prepared gelatin plates. Swirl the mixture to ensure it is spread evenly across the surface. Allow to gel in the 4 °C fridge O/N (allow to gel at least 8 hr before use). The substrates must be used within 3 days.
2. Alginate Oxidation
- Oxidize the sodium alginate bioink following the method for partially oxidized alginate by Bouhadir et al30 described below.
- To make a 5% oxidized alginate solution, dissolve 1 g of sodium alginate in 100 ml of distilled water. Add an aqueous solution of sodium periodate (0.25 M, 0.25 mmol), the oxidizing agent, to produce a 5% oxidation solution. Stir for 19 hr at RT. Add 40 ml ethylene glycol to the solution after 24 hr to end the reaction.
- Dissolve 2.5 g of sodium chloride in the solution. Add an excess amount of ethyl alcohol (2:1 ratio) to precipitate the oxidized alginates. Centrifuge the solution at 1,000 x g to collect the precipitates and re-dissolve them in distilled water. Repeat the ethanol wash.
- Freeze-dry the oxidized alginate pellets and store at -20 °C until ready for use.
- Determine the degree of oxidation by measuring the percentage of sodium periodate consumed before being terminated by the ethylene glycol.
- Prepare a potassium iodide solution (20% w/v, pH 7.0 sodium phosphate buffer) and a thyodene solution (10% w/v, pH 7.0 sodium phosphate buffer). Mix the two solutions with the oxidized alginate at RT.
- Gradually drop the reacted alginate and sodium periodate solution into the mixture of potassium iodide and theodyne solutions. Measure the absorbance of the mixture spectrophotometrically at 426 nm. When it has reached a maximum, record the used volume of alginate and sodium periodate solution as V1.
- The reaction is
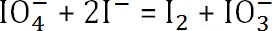 . The amount of unreacted sodium periodate is
. The amount of unreacted sodium periodate is 
- Subtract the amount of unreacted sodium periodate from the original concentration to determine the amount of sodium periodate consumed. Using the previous formula, determine the final oxidation degree of the alginate.
3. Alginate Peptide Conjugation
- Conjugate ligands with an exposed arginine-glycine-aspartate sequence ( peptide) into the previously prepared oxidized alginate by following the RGD-Alginate conjugation method by Rowley et al31described below to promote cell attachment and spreading.
- Use aqueous carbodiimide chemistry with G4RGDSPto conjugate31.
- Dissolve 1 g of 5% oxidized alginate in a 0.1 M 2-(N-morpholino) ethanesulfonic acid (MES) buffer, pH = 4. Add 1-ethyl-(dimethylaminopropyl) carbodiimide (EDC, 0.54 mmol) and N-Hydroxysuccinimide (NHS, 0.27 mmol) at 2:1 ratio to form amide intermediate.
- Add 0.28 mmol peptide, coupling to the backbone of the alginate polymer via the terminal amine. Stir at RT O/N.
- Stop the coupling reaction by adding 2.5 g sodium chloride to the solution. Add an excess amount of ethyl alcohol (2:1 ratio) to precipitate the oxidized alginates. Centrifuge the mixture at 4,000 x g for 5 min to collect the precipitates. Aspirate the media in the cell culture hood and re-dissolve the precipitates in distilled water. Repeat the ethanol wash.
- Freeze-dry the precipitates until it becomes completely dried (will appear as a white powdery substance) and store in the -20 °C fridge for later use.
4. Human Adipose Tissue Stromal Cells (hADSC’s) Cell Culture
- Culture human adipose tissue stromal cells (hADSC’s) in 75 cm treated cell culture flasks (T75 flasks), covered with 15 ml low glucose DMEM with 10% fetal bovine serum and 1% penicillin-streptomycin, 1% glutamine, and 1% antimycin. Change the media, in the cell culture hood, every two days until they have reached confluency (80-90%).
- Once confluent, transfer the T75 flasks to the cell culture hood and suspend the hADSC’s using the trypsin enzyme digestion method.
- In the hood, aspirate all of the cell culture media off of the cells. Rinse with 5 ml of Dulbecco’s Phosphate-Buffered Saline with calcium and magnesium (DPBS ++). Aspirate the DPBS++ off of the cells.
- While in the hood, make a solution of trypsin and DPBS++ by mixing 1 ml trypsin and 4 ml DPBS++. Each flask requires 5 ml of the solution, so make the appropriate volume for the number of confluent flasks. Add 5 ml of the trypsin/DPBS++ to each flask and put them in the incubator for 2 min.
- After 2 min, remove the flasks and lightly tap the sides of them to loosen the cells from the bottoms. Look at each flask under a microscope to ensure the cells are suspended. Place the flasks back in the cell culture hood and add 3 ml of appropriate cell culture media to each flask. This ends the trypsin reaction.
- Transfer the cell-laden media from each flask and put in a 50 ml conical. Centrifuge them at 1,000 x g for 5 min. The cells should appear as a little white pellet in the bottom of the conical. Transfer back to the cell culture hood and aspirate the media. Resuspend the cells in 2 ml of cell culture media.
- Count the cells using a hemocytometer under the microscope. Once the cells have been counted, in the culture hood, aliquot the amount of media containing ~1.3 million cells and transfer to a 15 ml conical. Centrifuge the 15 ml conical containing the cells again for 5 min at 1,000 x g.
- In the culture hood, reseed the remaining cells in multiple T-75 flasks, adding a concentration of ~350,000 cells to each flask. Add 15 ml of DMEM media and return to the incubator until confluent again.
- Once the centrifuge cycle is complete, return the 15 ml conical to the cell culture. Aspirate the media from the cell pellet, and resuspend the cells in aqueous alginate solution at a concentration of 1.3 million cells per milliliter of bioink, terteriating the solution often so there is a homogeneous distribution of cells throughout the bioink. Load the cell-laden solution into a sterile printer-compatible 3 ml syringe and screw on the sterile 22 G plastic tip.
5. Bioprinter Setup
- Turn on the bioprinter, each of the dispenser computers, and the recirculating water bath.
- Manually set the recirculating water bath temperature to for the gelation mechanism.
- Manually set printing parameters for each dispenser on the correlating dispenser computer. Set the dispense volume to 230 nl, number of backsteps to 0, and the dispense rate to 10μl –sec.
- Open the design software and the program for viewing the USB camera’s display on the computer.
- Using the software, manually enter the coordinates for a 5 x 5 dot array with 2.4 mm spacing between drops.
- Set the printing parameters to be: distance between tip end and substrate surface = 0.1 mm; height syringe is lifted between depositions = 20 mm; the amount of time per deposition = 1 sec.
- Save the program and send it to the robot.
- Place the gelatin/TiO2-containing Petri dish on the 4 °C printer stage. Close and lock the chamber door.
- Use the PLC to initialize the ultraviolet light sources, and sterilize the chamber for 90 sec.
- Once sterilization is complete, open the chamber and load the syringe containing hADSC’s suspended in alginate into Gun 1. Close and lock the chamber door.
- Use the PLC to turn on the fan system, wait 30 sec for equilibrium internal pressure.
- On the computer, run the program containing the geometrical pathway and printing parameters.
- Throughout the printing process, watch the USB camera’s display on the computer to confirm accurate and uniform printing.
- Once printing has finished, allow the constructs to gel for 40 min.
6. Cell Viability Assessment
- Cover the constructs that are not going to be imaged immediately post-printing in DMEM and store in the incubator until time of imaging.
- To quantify the viability of the constructs, stain them using a fluorescent-based viability/cytotoxicity assay, and image using confocal microscopy.
- Following the kit instructions, prepare a staining solution containing calcein AM and ethidium homodimer-1. To make 10 ml of staining solution, add 20 μl of the ethidium homodimer-1 and 5 μl of the calcein am to 10 ml of sterile, tissue culture-grade Dulbecco’s Phosphate-Buffered Saline (+magnesium, +calcium; DPBS++).
- Immerse the bioprinted constructs in the stain solution for 15 min in the dark.
- Image the stained constructs using a confocal microscope system at days 0 and 8. Take multiple pictures of each bioprinted construct, using Z-stack parameters of 30 optical slices over a 300 μm depth, and manually count the cells. If cells appear yellow or green count them as alive, and if red, count them as dead.
- Calculate the cell viability percentage as the number of live cells divided by the total number of cells in the construct; Cell Viability = number of live cells (green+yellow)/ number of total cells (green+yellow+red) x 100%.
- Calculate the amount of cell proliferation for each sample as the cell number of day 8 divided by the cell number on day 0; Cell Proliferation = live cell count on day 8/ live cell count on day 0 x 100%.
7. RGD Peptide Conjugation Analysis
- To analyze the success of RGD peptide conjugation on the alginate, compare RGD-conjugated alginate and non-conjugated alginate. To do this, image the printed constructs using (4’, 6-Diamidino-2-Phenylindole, Dihydrochloride) (DAPI) and phalloidin stains.
- Make the phalloidins working solution by diluting 5 μl of the methanolic stock solution with 200 μl of DPBS++. Store at -20 °C until use.
- Make a 300 μM stock solution of the DAPI stain following the equation: (0.10509 g/L)/(350.3 g/mol)=3 × 10-4 M=0.0003 M=0.300 mM=300 μM. Make the DAPI working solution by diluting the stock solution 1:100 in DPBS++ to obtain 3 μM solution. Store at -20 °C until use.
- Completely submerge the sample in 4% paraformaldehyde. Incubate for 1 hr at RT. Wash three times with DPBS++, allowing the solution to sit for 5 min each wash. Transfer the gel sample from the well to a glass slide, flipping the gel over in the process. Immerse the gel in 0.1% Triton X-100 (0.1 g/ 100 ml) in DPBS++ for 10 min. Wash three times with DPBS++, allowing 5 min for each wash.
- Stain the printed constructs with phalloidin by immersing them in the working solution. Cover with foil and incubate for 4 hr. Remove the phalloidin stain and wash three times with DPBS++. The first wash should be fast, the latter washes should sit for 5 min each.
- Stain the printed constructs with DAPI by immersing them in the DAPI working solution. Cover with foil and incubate at RT for 30 min. Wash three times with DPBS++, allowing each wash to sit for 5 min. Observe and image the samples on a confocal microscope system.
Wyniki
The results demonstrate the bioprinter is capable of depositing cell-laden hydrogels in specific three-dimensional locations accurately and consistently using computer-aided software. These softwares determine the placement of each droplet and control many of the parameters for dispensing (Figure 3,4). The repeatability of the bioprinter to appropriately deposit biomaterials is fundamental to its success in tissue engineering applications.
Cell viability, one of the requiremen...
Dyskusje
The primary focus of tissue engineering is to bridge the gap between organ shortages and transplantation needs by developing biological substitutes capable of restoring, maintaining, or improving native tissue functio. This has led to the direct fabrication of scaffolds with a complex, anatomically correct external geometry, and precise control over the internal geometr. Three-dimensional bioprinting is a methodology used for generating three-dimensional constructs of various sizes and shapes from a digital model using a...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by Government Support under Grant No. EPS-0903795 awarded by the National Science Foundation, NIH NIDCR R01-DE019355 (MJY PI), and Grant 8P20 GM103444 (YM PI).
Materiały
| Name | Company | Catalog Number | Comments |
| Positioning Robot (JR2000 XYZ) | Janome | ||
| Dispensers: SDAV Linear Drive SmartDispensers | Fishman Corporation | ||
| Optical Light Sensors: | Keyensce | ||
| Displacement Laser: OD Mini | SICK | ||
| Recirculating Water Bath: Polystat | Cole-Parmer | EW-12122-02 | |
| USB Cameras: Dino-Lite Premier 5MP | AnMo Electrionics/YSC Technologies | AD7013MT | |
| Printer-Compatible Computer Design Software: JR-C Points | Janome | Comes with purchase of Janome Robot | |
| Computer-Aided Design Drawing Software: Visual PathBuilder | RatioServ | Can be downloaded at: www.ratioserv.com/index.php/downloads | |
| Printer 3 cc Syringes: | Fishman Corporation | 122051 | |
| 22 G Dispenser Tips | Fishman Corporation | Z520122 | |
| Calcium Chloride Dihydrate | Sigma-Aldrich | 10035-04-8 | |
| Sodium Chloride | Sigma-Aldrich | 7647-14-5 | |
| Porcine Gelatin | Sigma-Aldrich | 9000-70-8 | |
| Titanium Dioxide | Sigma-Aldrich | 13462-67-7 | |
| Protanal LF 20/40 Alginate (Sodium Alginate) | FMC BioPolymer | 9005-38-3 | |
| Hydrochloric Acid | Sigma-Aldrich | 7647-01-0 | |
| Ethylene Glycol | Mallinckrodt Baker, Inc | 9300-01 | |
| Sodium Periodate | Sigma-Aldrich | 7790-28-5 | |
| hADSC | Lonza | PT-5006 | Store in vials in liquid nitrogen until use. |
| Dulbecco's Modified Eagle's Medium | Gibco Life Technologies | 11965-092 | Warm in 37 °C water before use. |
| Trypsin/EDTA | Lonza | CC-5012 | Warm in 37 °C water before use. |
| Calcein AM | Gibco Life Technologies | C3100MP | Store in the dark at -80 °C until use. |
| Live/Dead Mammalian Viability Assay Kit | Invitrogen Life Technologies | L-3224 | Store in the dark at -80 °C until use. |
| MES Hydrate | Sigma-Aldrich | M2933 | |
| N-Hydroxysuccinimide | Sigma-Aldrich | 130672 | |
| 1-ethyl-(dimethylaminopropyl) carbodiimide (EDC) | Sigma-Aldrich | E1769 | 10 G |
| Dulbecco's Phosphate-Buffered Saline, +Calcium, +Magnesium | Life Technologies | 14040133 | Warm in 37 °C water before use. |
| Dulbecco's Phosphate-Buffered Saline, -Calcium, -Magnesium | Life Technologies | 14190144 | Warm in 37 °C water before use. |
| RGD Peptides | International Peptides | ||
| Alexa Fluor 546 Phalloidin Stain | Invitrogen Life Technologies | A22283 | Store at -20 °C until use |
| (4’, 6-Diamidino-2-Phenylindole, Dihydrochloride) (DAPI) Stain | Life Technologies | R37606 | Store at -20 °C until use |
Odniesienia
- Langer, R., Vacanti, J. P. . Tissue Engineering. Science. 260 (5110), 920-926 (1993).
- Derby, B. Review: Printing and Prototyping of Tissues and Scaffolds. Science. 338 (6109), 921-926 (2012).
- Kachurin, A. M., et al. Direct-Write Construction of Tissue-Engineered Scaffolds. Mat. Res. Soc. Symp. Proc. 698, 10-1557 (2002).
- Sachlos, E., Czernuszka, J. T. Making Tissue Engineering Scaffolds Work. Review on the Application of Solid Freeform Fabrication Technology to the Production of Tissue Engineering Scaffolds. European Cells and Materials. 5, 29-40 (2003).
- Yeong, W. Y., Chua, C. K., Leong, K. F. Rapid Prototyping in Tissue Engineering. Challenges and Potential. Trends Biotechnol. 22 (12), 643-652 (2004).
- Landers, R., Pfister, A., Hubner, U., John, H., Schmelzeisen, R., Mulhaupt, R. Fabrication of Soft Tissue Engineering Scaffolds by means of Rapid Prototyping Techniques. Journal of Materials Science. 37 (15), 3107-3116 (2002).
- Murphy, S. V., Skardal, A., Atala, A. Evaluation of Hydrogels for Bio–Printing Applications. Journal of Biomedical Materials Research Part A. 101A (1), 272-284 (2013).
- Burg, K. J. L., Boland, T. Minimally Invasive Tissue Engineering Composites and Cell Printing. IEEE Eng Med Biol Mag. 22 (5), 84-91 (2003).
- Billiet, T., Vandenhaute, M., Schelfhout, J., Van Vlierberghe, S., Dubruel, P. A Review of Trends and Limitations in Hydrogel-Rapid Prototyping for Tissue Engineering. Biomaterials. 33 (26), 6020-6041 (2012).
- Khalil, S., Nam, J., Sun, W. Multi–Nozzle Deposition for Construction of 3D. Biopolymer Tissue Scaffolds. Rapid Prototyping Journal. 11 (1), 9-17 (2005).
- Pataky, K., Braschler, T., Negro, A., Renaud, P., Lutolf, M. P., Brugger, J. Microdrop Printing of Hydrogel Bioinks into Three–Dimensional Tissue–Like Geometries. Adv Mater. 24 (3), 391-396 (2011).
- Pati, F., Shim, J. H., Lee, J. S., Cho, D. W. Three-Dimensional Printing of Cell–Laden Constructs for Heterogeneous Tissue Regeneration. Manufacturing Letters. 1 (1), 49-53 (2013).
- Gruene, M., et al. Laser Printing of Three–Dimensional Multicellular Arrays for Studies of Cell–Cell and Cell–Environment Interactions. Tissue Eng. 17 (10), 973-982 (2011).
- Khalil, S., Sun, W. Bioprinting Endothelial Cells With Alginate for 3D Tissue Constructs. J Biomed Eng. 131 (11), 1-8 (2009).
- Xu, T., et al. Hybrid Printing of Mechanically and Biologically Improved Constructs for Cartilage Tissue Engineering Applications. Biofabrication. 5 (1), 1-10 (2012).
- Zhang, T., Yan, K. C., Ouyang, L., Sun, W. Mechanical Characterization of Bioprinted in vitro Soft Tissue Models. Biofabrication. 5 (4), 1-10 (2013).
- Chung, J. H. Y., et al. Bio–ink Properties and Printability for Extrusion Printing Living Cells. J. Biomater. Sci., Polym. Ed. 1 (7), 763-773 (2013).
- Yang, S., Leong, K. F., Du, Z., Chua, C. K. The Design of Scaffolds for Use in Tissue Engineering. Part II. Rapid Prototyping Techniques. Tissue Engineering. 8 (1), 1-11 (2002).
- Ferris, C. J., Gilmore, K. G., Wallace, G. G., Panhuis, M. Biofabrication: An Overview of the Approaches Used for Printing of Living Cells. Appl. Microbiol. Biotechnol. 97 (10), 4243-4258 (2013).
- Lu, L., Mikos, A. G. The Importance of New Processing Techniques in Tissue Engineering. MRS Bull. 21 (11), 28-32 (1996).
- Wake, M. C., Gupta, P. K., Mikos, A. G. Fabrication of pliable biodegradable polymer foams to engineer soft tissues. Cell Transplant. 5, 465-473 (1996).
- Mironov, V., Visconti, R. P., Kasyanov, V., Forgacs, G., Drake, C. J. Organ Printing: Tissue Spheroids as Building Blocks. Biomaterials. 30 (12), 2164-2174 (2009).
- Norotte, C., Marga, F. S., Niklason, L. E. Scaffold–free Vascular Tissue Engineering Using Bioprinting. Biomaterials. 30 (30), 5910-5917 (2009).
- Devillard, R., et al. Cell Patterning by Laser–Assisted Bioprinting. Methods Cell Biol. 119, 159-174 (2014).
- Binder, K. W., Allen, A. J., Yoo, J. J. Drop–on–Demand Inkjet Bioprinting: a Primer. Gene Ther Reg. 6 (1), 33 (2011).
- Xu, T., et al. Viability and Electrophysiology of Neural Cell Structures Generated by the Inkjet Printing Method. Biomaterials. 27 (19), 3580-3588 (2006).
- Calvert, P. Inkjet Printing for Materials and Devices. Chem Mater. 13 (10), 3299-3305 (2001).
- Chang, C. C., Boland, E. D., Williams, S. K. Direct–Write Bioprinting Three–Dimensional Biohybrid Systems for Future Regenerative Therapies. J Biomed Mater Res B Appl Biomater. 98 (1), 160-170 (2011).
- Li, M. G., Tian, X. Y. A Brief Review of Dispensing–Based Rapid Prototyping Techniques in Tissue Scaffold Fabrication: Role of Modeling on Scaffold Properties Prediction. Biofabrication. 1 (3), 1-10 (2009).
- Bouhadir, K. H., Lee, K. Y., Alsberg, E., Damm, K. L., Anderson, K. W., Mooney, D. J. Degradation of Partially Oxidized Alginate and its Potential Application for Tissue Engineering. Biotechnol Prog. 17 (5), 945-950 (2001).
- Rowley, J. A., Madlambaya, G. Alginate Hydrogels as Synthetic Extracellular Matrix Materials. Biomaterials. 20 (1), 45-53 (1999).
- Smith, C. M., Christian, J. J., Warren, W. L. Characterizing Environmental Factors that Impact Viability of Tissue–Engineered Constructs Fabricated by a Direct–Write Bioassembly Tool. Tissue Engineering. 13 (2), 373-383 (2007).
- Ozbolat, I., Yu, Y. Bioprinting Towards Organ Fabrication: Challenges and Future Trends. IEEE Trans Biomed Eng. 60 (3), 691-699 (2012).
- Peltola, S. M., Melchels, F. P., Grijpma, D. W., Kellomaki, M. A. A Review of Rapid Prototyping Techniques for Tissue Engineering Purposes. Annals of Medicine. 40 (4), 268-280 (2008).
- Malda, J., et al. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv Mat. 25 (36), 5011-5028 (2013).
- Murphy, S. V., Atala, A. 3D Bioprinting of Tissues and Organs. Nat Biotech. 32 (8), 773-785 (2014).
- Jia, J., et al. Engineering Alginate as Bioink for Bioprinting. Acta Biomaterialia. 10 (10), 4323-4331 (2014).
- Forty, R. A., Steinberg, M. S. The Differential Adhesion Hypothesis: a Direct Evaluation. Developmental Biology. 278 (1), 255-263 (2005).
- Wang, L., Shansky, J., Borselli, C., Mooney, D., Vandenburgh, H. Design and Fabrication of a Biodegradable, Covalently Crosslinked Shape–Memory Alginate Scaffold for Cell and Growth Factor Delivery. Tis Eng Part A. 18 (19-20), 2000-2007 (2012).
- El–Sherbiny, I. M., Yacoub, M. H. Hydrogel Scaffolds for Tissue Engineering: Progress and Challenges. Global Cardiology Science, & Practice. 3 (38), 316-342 (2013).
- Smith, C. M., et al. Three–Dimensional BioAssembly Tool for Generating Viable Tissue-Engineered Constructs. Tissue Engineering. 10 (9–10), 1566-1576 (2004).
- Ozbolat, I. T., Chen, H. Development of a ‘Multi-arm Bioprinter’ for Hybrid Fabrication of Tissue Engineering Constructs. Robotics and Computer–Integrated Manufacturing. 30 (3), 295-304 (2014).
- Kolesky, D. B., Truby, R. L., Gladman, A. S., Busbee, T. A., Homan, K. A. Three-Dimensional Bioprinting of Vascularized, Heterogeneous Cell–Laden Tissue Constructs. Adv Mater. X. Adv Mater. X, x-y (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone