Method Article
Transcutaneous Assessment of Renal Function in Conscious Rodents
W tym Artykule
Podsumowanie
Determination of glomerular filtration rate (GFR) is the gold standard to assess overall kidney function. However, traditional procedures to measure this parameter are cumbersome and require a large investment of time. Here we describe a faster and minimally invasive method to determinate GFR transcutaneously.
Streszczenie
Glomerular filtration rate (GFR) is the gold standard to assess overall kidney function. However, traditional methods to evaluate GFR are cumbersome and time-consuming. In addition, serial blood or urine samples are required, with the associated stress for the experimental animals. A recent technique significantly reduces the investment in time and resources, minimizing the invasiveness and the animal stress, but being equally valid as the traditional approaches. The method measures transcutaneously renal function. Using an optical device and the exogenous renal marker fluorescein isothiocyanate (FITC)-sinistrin, this technique is capable of measuring the elimination kinetics of the marker through the skin. With neither blood nor urine samples nor the associated laboratory assays needed, the results of the transcutaneous measurement are almost instantaneously available. The method has been already validated in different species and successfully applied in several models of renal pathology. Moreover, due to its minimally invasive characteristics, it is suitable for sequential measurements within the same animal. Here is provided a detailed protocol to carry out the transcutaneous assessment of renal function in rodents.
Wprowadzenie
Glomerular filtration rate (GFR) is the best parameter to assess overall renal function. The gold standard to determine GFR relies on urinary or plasma clearance of exogenous renal markers, such as inulin1. However, these procedures are time consuming and complicated, requiring the collection of serial blood and/or urine samples and posterior laboratory assays for its analysis. In addition, these methods are invasive and stressful for the animals, limiting the number of times and frequency in which the measurements can be repeated. A number of alternative approaches have been developed to simplify the classical procedures to determine GFR, but they still rely on urine and plasma sampling2-4 and/or require deep anesthesia5,6, which is known to influence renal hemodynamics and function7,8. End-products of metabolism, such as creatinine, are also widely used to estimate kidney function. However, it is known that the accuracy of these endogenous markers is not optimal and, moreover, to analyze them, urine or blood sampling is also indispensable.
Here we describe a transcutaneous methodology to assess renal function in conscious animals. This method is simpler and faster than traditional methods as well as only minimally invasive. With this technique, using a miniaturized optical device and the exogenous renal marker fluorescein isothiocyanate (FITC)-sinistrin, it is possible to determine kidney function almost in real-time without a need for plasma or urine sampling and without surgical catheterization for substance administration. Due to these characteristics, the method is suitable to perform sequential measurements in the same animal.
FITC-sinistrin is a renal marker freely filtered by the glomerulus, with no tubular reabsorption or secretion. The optical part of the miniaturized device consists of two light-emitting diodes (LEDs), which excite the administered marker and a photodiode to detect the fluorescence emitted through the skin. The recorded data are stored in the internal memory integrated in the device and they can be used to generate the FITC-sinistrin elimination kinetics curve. Power is supplied by a small rechargeable lithium battery. For more detailed information on the device and its individual components refer to Schreiber et al.9. The device is easily mounted on the animal body using a double-sided adhesive patch carrying a transparent window for the optical components. The results are readily available after the recording period is over.
With the method and protocol provided here, we introduce a promising technology which could replace the current gold standard to determine GFR, not only in research but also in the clinical field.
Protokół
Necessary experiments to develop the protocol were performed according to national regulations and were approved by the local ethical science committee (Regierungspräsidium Karlsruhe).
1. Preparation of FITC-sinistrin Injection Solution
- Dissolve FITC-sinistrin in physiological saline to prepare a stock solution. The recommended dose in mice is 7.5 mg/100 g body weight (BW), while in rats 5 mg/100 g BW FITC-sinistrin is preferable. For mice, prepare a solution of FITC-sinistrin stock solution at 15 mg/ml, while for rats prepare at a concentration of 40 mg/ml.
Note: FITC-sinistrin stock solution can be prepared in advance and stored at -20 °C protected from light.
2. Animal Preparation
- Acclimate animals for at least one week before their introduction in any experiment.
- Anesthetize the animal using 5% isoflurane at 5 L/min O2 flow for 2 min.
- Confirm that the animal is properly anesthetized by observing its breathing, which becomes slower and deeper, and verify unresponsiveness with a lack of a toe response.
- Once the animal is asleep, reduce the anesthesia to 2.5% isoflurane at 2 L/min O2 delivery rate and take the BW.
- Remove the fur from the flank of the back of the animal using an electric shaver. Shave an area slightly bigger than the area that will be occupied by the double-sided adhesive patch, which has dimensions of 3 cm x 6 cm.
- Subsequently apply depilation cream for a short period (2-3 min) to remove the remaining fur.
- Wash thoroughly the area until the cream has been completely removed, as the cream itself may be fluorescent.
Note: Avoid scratching the skin of the animal as it can produce irritation/edema. If the depilated area shows pigmentation on the skin, shave a bigger area until a non-pigmented spot for the optical part of the device is found. It is recommended to shave 24 hr in advance to minimize anesthesia exposure.
3. Device Preparation
- Place the optical device to measure renal function onto one side of the double-sided adhesive patch, positioning the optical part above the transparent window (Figure 1) leaving the opposite part with the protective foil.
Note: When working with small rodents, reduce the size of the patch as shown in the Figure 2. - Attach a piece of double-sided adhesive patch of matching size to the battery as shown in Figure 3.
4. Fixation of the Device on the Animal
- Calculate the appropriate volume of injection based on the animal body weight.
- Anesthetize the animal with isoflurane as previously indicated on sections 2.1-2.3.
- Cut a piece of tubular elastic gauze bandage of about 1 cm longer than the width of the double-sided adhesive patch to be used.
- Pull the elastic gauze bandage over the head of the animal and place on its back, leaving the shaved area uncovered.
Note: Alternatively, fix the device using only adhesive tape, but be aware of undue pressure on the device. - Connect the battery to the device by plugging the battery connector to the corresponding port on the device. After that remove the protective foil from the piece of patch and mount the battery on the upper surface of the device (Figure 4A). Ensure that the battery is properly plugged by checking that the device starts blinking.
Note: In rats, the battery can be also attached directly on the adhesive patch, next to the device (Figure 4B). - Remove the protective cover from the patch with the operating device and place it onto the shaved area on the back of the animal holding its edges until it is correctly fixed.
- Cover the device with the tubular elastic gauze bandage adhering it to the adhesive surface of the patch (Figure 5).
- Properly stretch the tubular bandage on the abdomen of the animal, ensuring that the limbs can move freely.
- For a better fixation and protection of the device, apply a strip of adhesive tape following the shape of the device and covering the wires of the battery.
- Measure background for 1 to 3 min, without putting pressure on the device
5. FITC-sinistrin Administration and Measurement Procedure
- If necessary, warm up the tail of the animal using warm water before injection or a temperature-controlled heating plate along all the procedure.
- Inject through tail vein the appropriate volume of FITC-sinistrin stock solution. The volume of injection depends on animal BW and therefore must be calculated for each individual considering the desired dose and concentration of stock solution, aforementioned in section 1.1.
- Ensure that all the FITC-sinistrin solution is administered intravenously and not subcutaneously.
Note: Alternatively, FITC-sinistrin can be administered using a pre-implanted intravenous catheter exited at the nape of the neck in rodents or via retro orbital injection in mice. - Carefully, return the animal to its home cage avoiding strong movements and any pressure on the device, as it would introduce movement artifacts.
- Place the home cage in a calm place to avoid that the animal is disturbed.
- Perform the measurement during at least 1 hr if working with mice and 2 hr when using rats. During that period, the optical device will measure through the skin the fluorescence emitted by FITC-sinistrin.
Note: During the recording period the animal must be hosted alone. Furthermore water supply, as well as protruding structures such as the wire lids, must be removed to avoid damaging of the electronic device and movement artifacts due to impacts with objects.
6. Device Removal
- Once the recording period is over, remove the device without anesthesia; however, if needed, anesthetize the animal briefly under 5% isoflurane at 5 L/min O2 delivery rate during 2 min.
- Carefully, pull off the adhesive tape first, then the tubular bandage.
- Gently detach the double-sided adhesive patch from the skin and return the animal to its normal home cage.
7. Read out of Data
- Disconnect the battery from the device and remove the adhesive patch.
Note: The data are stored on the device until a new measurement starts. When a battery is connected the stored data are overwritten with the new recordings. Therefore, do not reconnect the battery before downloading the data from the device. There is a 10 sec grace period intended for accidental reconnections of the battery. - Connect the device to a PC using a micro USB cable and download the data using the software provided. The output file is a .csv file that can be opened and modified with a spreadsheet program.
- Open the data file with the specific software for the optical device and generate the elimination kinetics curve using the software protocols.
- Analyze the curve by following the instructions provided with the optical device. Shortly, for the evaluation of the data, set the background signal measured prior FITC-sinistrin administration and mark the beginning of the exponential excretion phase of the marker, which usually occurs 15 min and 45 min after the bolus injection, in mice and rats respectively.
Note: The software will automatically display the FITC-sinistrin half-life (t1/2) along with an R2 value, determined by a 1-compartment model. t1/2 can be utilized to calculate GFR by using a conversion factor9,10.
Wyniki
The setup of the transcutaneous measurement is very simple and fast: the device is placed on the double-sided adhesive patch (Figure 1) and adjusted in size if necessary (Figure 2), the battery is prepared (Figure 3) and connected (Figure 4).
This method to assess renal function has been already validated in different species by comparison with the traditional approach of plasma clearance9,11,12. Following the protocol exposed here, Schreiber et al. demonstrated the validity of the technique in different mouse models, showing highly comparable results among the transcutaneous measurement and plasma clearance for all the groups studied (Table 1)9. In this work the obtained t1/2 was converted to GFR using a mouse specific semi-empirical conversion factor.
The consistency of the transcutaneous assessment of renal function has been also proved using different strains of mice. Sequential measurements within 3 days in the same animal showed a coefficient of variances of 3.0-6.2%13. In this study there was no conversion to GFR but the results were expressed and interpreted directly in terms of t1/2.
The possibility to have the results almost in real-time is one of the great advantages of this method. After the recording period, the results are immediately available for analysis and the provided software displays the excretion curve of FITC-sinistrin instantaneously (Figure 6). Within the same software the t1/2 of FITC-sinistrin can be obtained, which can be directly used as the parameter to evaluate renal function of converted in terms of GFR. Figure 7 shows how it looks the FITC-sinistrin excretion curve measured transcutaneously in an animal with renal impairment. When renal function is affected, FITC-sinistrin t1/2 increases due to the reduced excretion of the substance and the appearance of the curve changes. Typically, the measured curve does not come back to background level and presents an increased area under the curve. In the same animal, measurements from pre- to post-injury can experience an increase in the maximum fluorescence intensity due to accumulation of the marker caused by its reduced excretion. In presence of kidney failure, the transcutaneously measured curve of FITC-sinistrin can show a steady state due to severely impaired function (Figure 7B).
The use of the miniaturized device in several strains of rodents with different health status has shown that this technique is suitable and sensitive enough to detect changes due to renal disease and ageing. Table 2 shows a summary of the murine models studied to date with this method.

Figure 1. Placement of the device on the double-sided adhesive patch. The device is mounted on the adhesive patch positioning its optical part in the transparent window.

Figure 2. Adapting the adhesive patch for small-sized rodents. For use in small animals it is recommended to reduce the size of the patch. A: Use the device as a guide to cut the patch properly. B: Once the desired size has been obtained, the device can be placed on the sticky surface of the patch. Please click here to view a larger version of this figure.
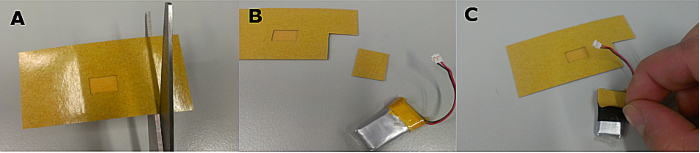
Figure 3. Preparation of the battery. To attach the battery to the device, cut a small piece of double-sided adhesive patch (A and B) and place it on the surface of the battery (C). Please click here to view a larger version of this figure.
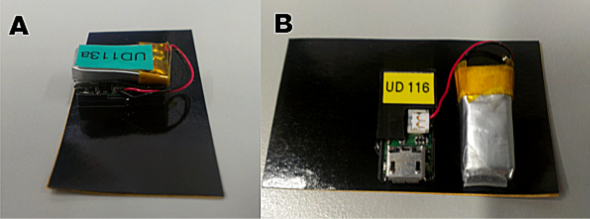
Figure 4. Connection and placement of the battery during the measurement. (A) In small rodents such as mice, due to the reduced space, the battery must be placed on top of the device. (B) In larger animals the battery can be placed next to the device. Please click here to view a larger version of this figure.
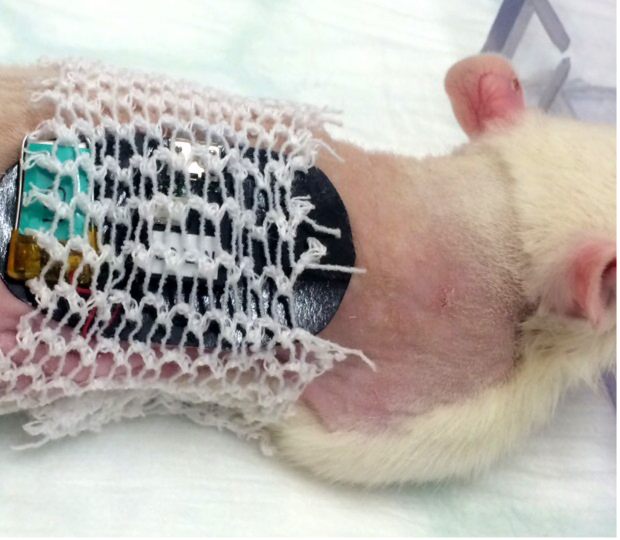
Figure 5. Placement of the tubular elastic gauze bandage in a rat. The tubular bandage should cover the double-sided adhesive patch without interfering with the free movement of the limbs for the comfort of the animal. Please click here to view a larger version of this figure.

Figure 6. Representative image of an elimination curve of FITC-sinistrin. The signal generated by FITC-sinistrin is detected transcutaneously and stored in the internal memory of the device. When the recorded data are downloaded onto a PC, the software generates a curve comparable to the one presented in the image. Y-axis shows the recorded fluorescence intensity [AU], emitted by the injected FITC-sinistrin marker, while X-axis represents the duration of the measurement in time [min]. Please click here to view a larger version of this figure.
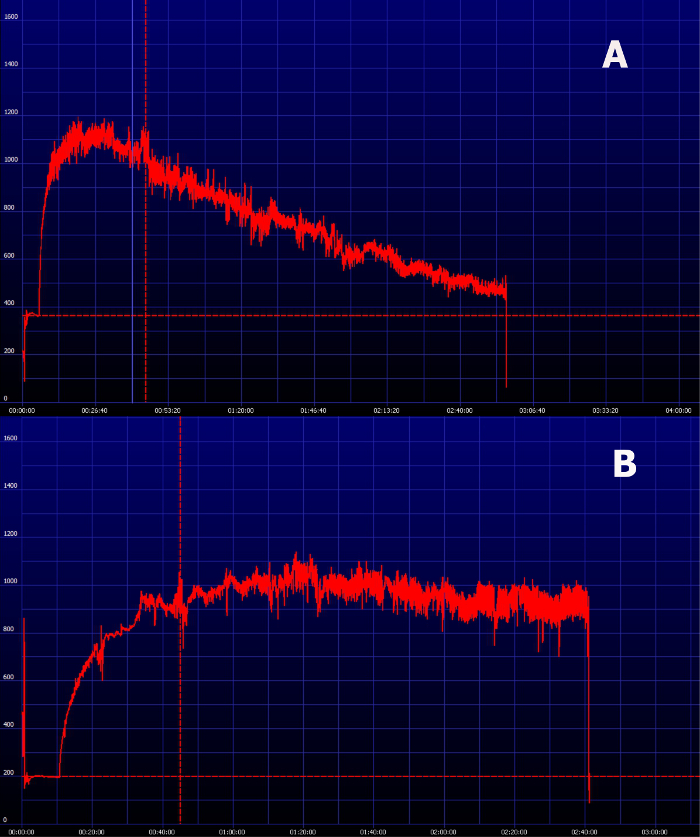
Figure 7. Representative image of an elimination curve of FITC-sinistrin in animals with impaired renal function. (A) FITC-sinistrin elimination curve in animals with reduced renal function typically shows an increased area under the curve and inability to reach the baseline within the normal measurement period. (B) In severely impaired animals the transcutaneously measured curve can show a steady state which indicates renal failure. Please click here to view a larger version of this figure.

Table 1. Validation of the transcutaneous measurement by comparing with the traditional plasma clearance. The transcutaneous measurement of renal function has been validated in different mouse models (healthy and unilaterally nephrectomized (UNX) C57BL/6-129 SV mice and mouse model of nephronophthisis (pcy)) by comparison with plasma clearance. Values are means ± SD. GFR, glomerular filtration rate9.
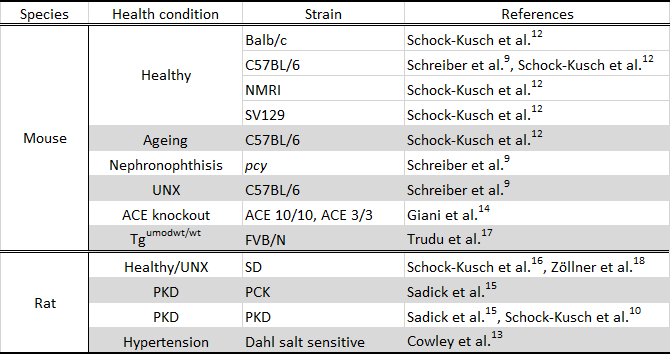
Table 2. Murine models studied using the transcutaneous measurement. UNX, unilaterally nephrectomized; ACE, angiotensin-converting enzyme; Tgumodwt/wt, transgenic uromodulin knockout, SD, Sprague Dawley rat; PKD, polycystic kidney disease.
Dyskusje
The present paper describes the procedure to determine kidney function in conscious rodents using a miniaturized device and the exogenous renal marker FITC-sinistrin. As stated before, this method has several advantages in comparison with the traditional procedures of urinary and plasma clearance. One of them is the independence from blood and urine sampling which leads to great savings in terms of consumables, time and, of course, animal stress.
As shown in Table 2, the exogenous renal marker FITC-sinistrin and the measurement of its elimination kinetics through the skin allow the impact of renal pathologies on kidney function to be studied 9,10,14-18. The serial collection of blood samples required to assess plasma clearance or biomarkers of renal disease are stressful for the animal, cumbersome and time-consuming for the researchers. Therefore, sequential measurements within the same animal have been limited by the welfare of the animals and methodological reasons. The proved within-animal reproducibility of the transcutaneous measurement13 makes it an ideal method for detecting changes in kidney function due to disease progression or ageing. For the same reason, it can also be extremely useful to study the effectiveness of therapeutic approaches.
Two critical steps for a successful application of this technology are the fixation of the optical device on the animal and the correct administration of the marker. The device must be appropriately fixed to avoid movement artifacts. For that purpose, preventing the attachment and detachment of the double-sided adhesive patch is crucial as it can lead to the loss of its adhesive properties and ultimately an inappropriate fixation on the animal. As mentioned in the protocol, immobilization and protection of the optical device by covering it with adhesive tape is an effective approach to ensure an adequate fixation. When performing FITC-sinistrin bolus injection it is crucial to administer all the substance intravenously and not subcutaneously. The release of the marker in the subcutaneous tissue can be easily identified by a yellow ring circling the tail of the animal produced by FITC-sinistrin's own color. A subcutaneous injection typically manifests as a flatter curve and prolonged marker excretion time. The possible limitation of this method is more related to the marker used than with the technology per se. When an edema is present in the skin of an animal, the transcutaneous assessment of renal function would not be recommended, as there is an additional compartment which could alter the excretion dynamics of FITC-sinistrin. Similarly, pigmented skin can limit the use of the technique due to the overlapping excitation and emission spectra of FITC-sinistrin and melanin. However, in animals with skin pigmentation in patches, the problem is solved by placing the optical part of the device in an unpigmented area.
It is possible to modify different aspects of the transcutaneous assessment of renal function in order to adapt the procedure to different animal species and/or approaches. For example, the recommended FITC-sinistrin dosage or the length of the measurement can be adjusted depending on the goal of the experiment as well as for different species or animal models. This adaptability of the method has already been confirmed by its successful application in different species of rodents and several types of strains with varied health conditions. In addition, the transcutaneous assessment of renal function has been recently validated in dogs and cats12 with excellent results. Due to the undoubted differences between these animals and rodents, this study determined the appropriate dose of FITC-sinistrin for both, dogs and cats, and it performed a much longer measuring time, 4 hours instead of the 1 or 2 hours used in rodents.
Taken together, the use of this technique can help us to gain more accurate insights in nephrology, including a better understanding of renal disease progression and the effect of therapies. The characteristics of the transcutaneous assessment of renal function places it as a suitable technique to be applied in human and veterinary clinical fields in the future, and should be considered as a promising technology to replace the traditional approaches.
Ujawnienia
NG holds a patent on the production of FITC-sinistrin.
Podziękowania
ZHP was supported by the Marie-Curie project: NephroTools.
Materiały
| Name | Company | Catalog Number | Comments |
| NIC-Kidney device | Mannheim Pharma & Diagnostics GmbH | Software, batteries and chargers are provided together with the device/s. Orderings should be done through info@mapdiagnostics.com | |
| FITC-sinistrin | Mannheim Pharma & Diagnostics GmbH | Orderings should be done through info@mapdiagnostics.com | |
| Double-sided adhesive patch | Mannheim Pharma & Diagnostics GmbH | Orderings should be done through info@mapdiagnostics.com | |
| Isis Rodent electric shaver | Braun Aesculap | GT420 | |
| Isoflurane | Abbott GmbH | PZN4831850 | |
| Leukosilk adhesive tape | BSN medical | 102200 | |
| Tubular elastic gauze bandage | MaiMed Medical GmbH | 73012 | There are different sizes available. Size 1 is recommended for mice. |
| Veet depilation cream | Reckitt Benckiser | PZN7768307 | Sensitive skin formulation is recommended as is more gentle with the skin of the animals |
| Micro USB cable | Samsung | APCBU10BBE | |
| Deltajonin Physiologic solution | AlleMan Pharma GmbH | 3366954 | Alternatively, it can be used saline or PBS |
Odniesienia
- Stevens, L. A., Levey, A. S. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 20, 2305-2313 (2009).
- Katayama, R., et al. Calculation of glomerular filtration rate in conscious rats by the use of a bolus injection of iodixanol and a single blood sample. J Pharmacol Toxicol Methods. 61, 59-64 (2010).
- Reinhardt, C. P., et al. Functional immunoassay technology (FIT), a new approach for measuring physiological functions: application of FIT to measure glomerular filtration rate (GFR). Am J Physiol Renal Physiol. 295, F1583-F1588 (2008).
- Rieg, T. A high-throughput method for measurement of glomerular filtration rate in conscious mice. J Vis Exp. , e50330(2013).
- Yu, W., Sandoval, R. M., Molitoris, B. A. Rapid determination of renal filtration function using an optical ratiometric imaging approach. Am J Physiol Renal Physiol. 292, F1873-F1880 (2007).
- Wang, E., Sandoval, R. M., Campos, S. B., Molitoris, B. A. Rapid diagnosis and quantification of acute kidney injury using fluorescent ratio-metric determination of glomerular filtration rate in the rat. Am J Physiol Renal Physiol. 299, F1048-F1055 (2010).
- Colson, P., et al. Does choice of the anesthetic influence renal function during infrarenal aortic surgery? Anesth Analg. 74, 481-485 (1992).
- Fusellier, M., et al. Influence of three anesthetic protocols on glomerular filtration rate in dogs. Am J Vet Res. 68, 807-811 (2007).
- Schreiber, A., et al. Transcutaneous measurement of renal function in conscious mice. Am J Physiol Renal Physiol. 303, F783-F788 (2012).
- Schock-Kusch, D., et al. Transcutaneous measurement of glomerular filtration rate using FITC-sinistrin in rats. Nephrol Dial Transplant. 24, 2997-3001 (2009).
- Schock-Kusch, D., et al. Transcutaneous assessment of renal function in conscious rats with a device for measuring FITC-sinistrin disappearance curves. Kidney Int. 79, 1254-1258 (2011).
- Steinbach, S., et al. A pilot study to assess the feasibility of transcutaneous glomerular filtration rate measurement using fluorescence-labelled sinistrin in dogs and cats. PLoS One. 9, e111734(2014).
- Schock-Kusch, D., et al. Reliability of transcutaneous measurement of renal function in various strains of conscious mice. PLoS One. 8, e71519(2013).
- Cowley, A. W. Jr, et al. Progression of glomerular filtration rate reduction determined in conscious Dahl salt-sensitive hypertensive rats. Hypertension. 62, 85-90 (2013).
- Giani, J. F., et al. Renal angiotensin-converting enzyme is essential for the hypertension induced by nitric oxide synthesis inhibition. J Am Soc Nephrol. 25, 2752-2763 (2014).
- Sadick, M., et al. Two non-invasive GFR-estimation methods in rat models of polycystic kidney disease: 3.0 Tesla dynamic contrast-enhanced MRI and optical imaging. Nephrol Dial Transplant. 26, 3101-3108 (2011).
- Trudu, M., et al. Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat Med. 19, 1655-1660 (2013).
- Zollner, F. G., et al. Simultaneous measurement of kidney function by dynamic contrast enhanced MRI and FITC-sinistrin clearance in rats at 3 tesla: initial results. PLoS One. 8, e79992(2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone