Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Soil Lysimeter Excavation for Coupled Hydrological, Geochemical, and Microbiological Investigations
W tym Artykule
Podsumowanie
This study presents an excavation method for investigating subsurface hydrological, geochemical, and microbiological heterogeneity of a soil lysimeter. The lysimeter simulates an artificial hillslope which was initially under homogeneous condition and had been subjected to approximately 5,000 mm of water over eight cycles of irrigation in an 18-month period.
Streszczenie
Studying co-evolution of hydrological and biogeochemical processes in the subsurface of natural landscapes can enhance the understanding of coupled Earth-system processes. Such knowledge is imperative in improving predictions of hydro-biogeochemical cycles, especially under climate change scenarios. We present an experimental method, designed to capture sub-surface heterogeneity of an initially homogeneous soil system. This method is based on destructive sampling of a soil lysimeter designed to simulate a small-scale hillslope. A weighing lysimeter of one cubic meter capacity was divided into sections (voxels) and was excavated layer-by-layer, with sub samples being collected from each voxel. The excavation procedure was aimed at detecting the incipient heterogeneity of the system by focusing on the spatial assessment of hydrological, geochemical, and microbiological properties of the soil. Representative results of a few physicochemical variables tested show the development of heterogeneity. Additional work to test interactions between hydrological, geochemical, and microbiological signatures is planned to interpret the observed patterns. Our study also demonstrates the possibility of carrying out similar excavations in order to observe and quantify different aspects of soil-development under varying environmental conditions and scale.
Wprowadzenie
Soil and landscape dynamics are shaped by the complex interaction of physical, chemical, and biological processes1. Water flow, geochemical weathering, and biological activity shape the overall development of the landscape into a stable ecosystem2,3. While surface changes are the most conspicuous features of landscape4, understanding cumulative effects of hydrological, geochemical, and microbiological processes in the subsurface region is crucial to understanding the underlying forces that shape a landscape2. Future climate perturbation scenarios further confound the predictability and pattern of landscape evolution5. It thus becomes a challenge to link small-scale processes to their large-scale manifestation on the landscape-scale6. Traditional short-run laboratory experiments or experiments in natural landscapes with unknown initial conditions and time-variable forcing fall short in capturing the intrinsic heterogeneity of landscape evolution. Also, due to strong nonlinear coupling, it is difficult to predict biogeochemical changes from hydrological modeling in heterogeneous systems7. Here, we describe a novel experimental method to excavate a fully controlled and monitored soil hillslope with known initial conditions. Our excavation and sampling procedure is aimed at capturing the developing heterogeneity of the hillslope along its length and depth, with the goal of providing a comprehensive dataset to investigate hydro-bio-geochemical interactions and their impact on soil formation processes.
Hydrologic systems found in nature are far from being static in time, with changes in hydrological responses taking place over a wide range of spatial and temporal scales3. The spatial structure of flow pathways along landscapes determines the rate, extent and distribution of geochemical reactions and biological colonization that drive weathering, the transport and precipitation of solutes and sediments, and the further development of soil structure. Thus, incorporating knowledge from pedology, geophysics, and ecology into theories and experimental designs to assess hydrologic processes and improve hydrologic predictions has been suggested8,9. Landscape evolution is also impacted by subsurface biogeochemical processes in conjunction with water dynamics, elemental migration during soil development, and by mineralogical transformations brought about by reaction of mineral surfaces with air, water, and microorganisms10. Consequently, it is important to study development of geochemical hotspots within an evolving landscape. Additionally, it is critical to relate geochemical weathering patterns to hydrological process and microbiological signatures during incipient soil formation in order to understand the dynamics of complex landscape development. The specific processes of soil genesis are governed by the combined influence of climate, biological inputs, relief and time on a specific parent material. This experiment was designed to address heterogeneities in the weathering of parent material governed by hydrological and geochemical variations associated with relief (including slope and depth) and the associated variability in microbial activity that is driven by environmental gradients (i.e., redox potential) under conditions where parent material, climate and time are held constant. With respect to microbial activity, soil microorganisms are critical components and have a profound impact on landscape stability11. They play a crucial role in soil structure, biogeochemical cycling of nutrients, and plant growth. Therefore, it is necessary to understand the significance of these organisms as drivers of weathering, soil genesis, and landscape formation processes, while simultaneously identifying the reciprocal effects of hydrological flow-paths and geochemical weathering on microbial community structure and diversity. This can be achieved by studying spatial heterogeneity of microbial community diversity over an evolving landscape whose hydrological and geochemical characteristics are also being studied in parallel.
Here, we present an excavation procedure of a soil lysimeter, operationally named miniLEO, designed to mimic the large-scale zero-order basin models of the Landscape Evolution Observatory (LEO) housed at Biosphere 2 (University of Arizona). The miniLEO was developed to identify small-scale landscape evolution patterns arising from cumulative heterogeneous hydro-bio-geochemical processes. It is a lysimeter 2-m in length, 0.5-m in width, and 1-m in height, and slope of 10° (Figure 1). Additionally, the walls of the lysimeter are insulated and coated with non-biodegradable two-part epoxy primer and an aggregate filled aliphatic urethane coat to avoid potential contamination or leaching of metals from the lysimeter frame into the soil. The lysimeter was filled with crushed basalt rock that was extracted from a deposit of late Pleistocene tephra associated with Merriam Crater in northern Arizona. The loaded basalt material was identical to the material used in the much larger LEO experiments. The mineral composition, particle size distribution, and hydraulic properties are described by Pangle et al.12. The downslope seepage face was lined with a perforated plastic screen (0.002-m diameter pores, 14% porosity). The system is fitted with sensors such as water content and temperature sensors, two types of water potential sensors, soil-water samplers, hydraulic weight balance, electrical conductivity probes, and pressure transducers to determine water table height. The lysimeter was irrigated for 18 months prior to the excavation.
The excavation was meticulous in its approach and was aimed at answering two broad questions: (1) what hydrological, geochemical, and microbial signatures can be observed across the length and depth of the slope with respect to simulated rainfall conditions and (2) whether relationships and feedbacks between hydro-bio-geochemical processes occurring on the hillslope can be deduced from the individual signatures. Alongside the experimental setup and excavation procedure, we present representative data and suggestions on how to apply similar excavation protocols for researchers interested in studying coupled earth-system dynamics and/or soil development processes.
Protokół
1. Devise a Sampling Matrix to Ensure Systematic and Comprehensive Sampling of Lysimeter
- Divide lysimeter into voxels of fixed length, width, and depth.
- Use a Euclidean space coordinate system and divide the total distance along each direction (X, Y and Z) into an adequate number of equally spaced intervals. Consider discarding the soil near the walls of the lysimeter to avoid boundary effects.
NOTE: A buffer of 5 cm along the four walls is adopted in this experiment to avoid boundary effects, while ensuring that the volume of soil collected is sufficient. - Assign each sample a unique XYZ location and identify as a voxel.
NOTE: In this excavation, X denotes the location along the width of the slope, Y denotes location along the length of the slope, while Z denotes location along the depth of the slope. The size of the intervals within each dimension determines the width, length, and depth of the voxels. Figure 2 shows the division of the lysimeter after determining spacing intervals along with the chosen origin for the XYZ system. The division in the current excavation scheme has 9 intervals along both Y and Z directions and 4 intervals along the X direction, producing a total of 324 voxels of 10 cm x 20 cm x 10 cm dimensions (Figure 3).
NOTE: The sampling strategy chosen ensures that the entire system is evenly sampled with minimal damage to the sensors. Boundaries of each voxel (1-2 cm) are discarded to limit cross contamination from neighboring voxels. Additionally, voxel dimensions ensure that enough soil material is available for microbiological, geochemical, and hydrological sample collection in each voxel.
- Use a Euclidean space coordinate system and divide the total distance along each direction (X, Y and Z) into an adequate number of equally spaced intervals. Consider discarding the soil near the walls of the lysimeter to avoid boundary effects.

Figure 1. Side-view of lysimeter. View of lysimeter from the seepage face. Also visible are three sensor regions (white PVC tubes) along the slope and sprinkler system at the four corners.
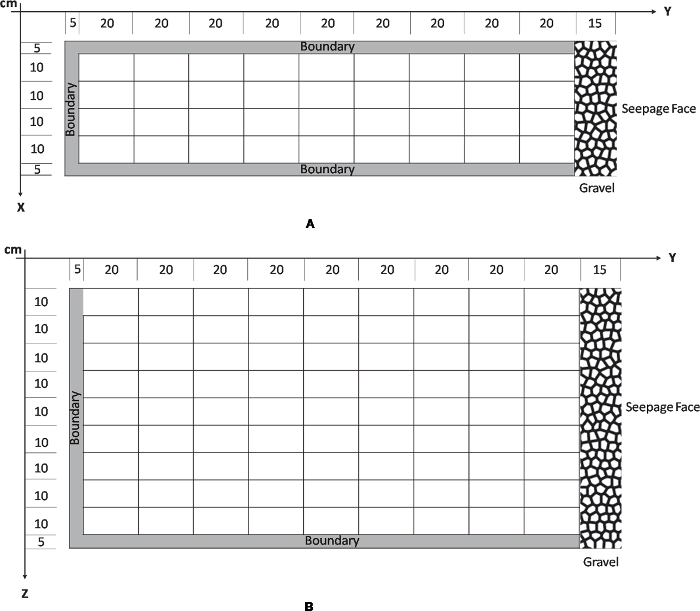
Figure 2. Sampling Scheme. Sampling scheme of lysimeter along XYZ. A. The X dimension divides the width into 4 sections each of 10 cm while Y divides the length into 20 cm. B. The Z dimension indicates depth and was divided into 9 layers of 10 cm depth. A boundary of 5 cm all along the edges of the lysimeter was identified to prevent collection of samples that can potentially exhibit boundary effect. Please click here to download this file.
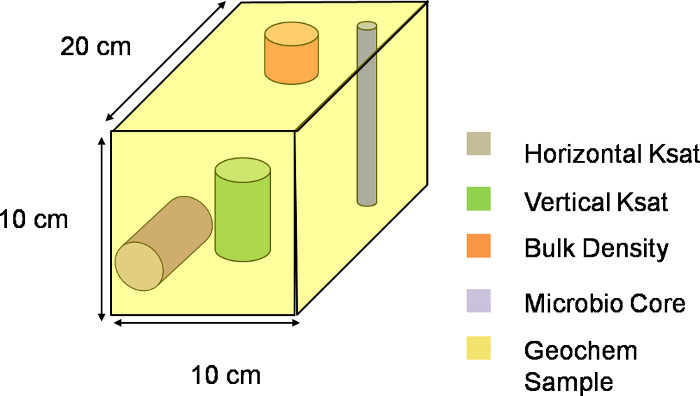
Figure 3. Three-dimensional representation of a voxel. Visual schematic of one voxel along the XYZ plane of the lysimeter. The entire slope was divided into 324 such voxels, with each voxel depicting a single sampling unit. Please click here to download this file.
2. Add Brilliant Blue FCF Dye to Track Water Infiltration in the Slope
- Apply brilliant blue dye at the surface of the soil, enough to cover top 105 cm of the surface along the Y direction. Cover the remaining soil with plastic sheets.
- Choose a concentration (here 10 g/L) to guarantee contrast against black basaltic soil. Add the dye to irrigation system tanks and dilute with water to the desired concentration.
- Decide the duration of the irrigation based on the desired depth of the infiltration front and the rate supplied by the irrigation system.
NOTE: For this study, an irrigation rate of 30 mm/hr for 20 min (Figure 4) prior to excavation is considered sufficient in order to identify heterogeneous patterns of water infiltration during the first few centimeters. - After dye application, give time for the infiltration to stop and the moisture states within the lysimeter to equilibrate. For this study, a period of 10 hr (overnight) between dye application and excavation was appropriate.
3. Demarcation of Voxels
- Attach measuring tape along the length of the slope to provide an in-situ reference system for guidance during demarcation of voxels.
- Mark the dimension of each soil voxel with the help of the measuring tape. Draw grid lines for each layer using aluminum-blade shields and plastic putty knives (Figure 4). Discard the boundary materials (5 cm from each wall to prevent boundary effects).

Figure 4. Top view of lysimeter. This view shows the dyed surface of layer 2 (10 cm deep). Grids drawn on the soil surface to aid sampling are also visible, along with core holes regions at each voxel after microbiological sample collection.
4. Microbiology Sample Collection
- Collect microbiology samples aseptically from each voxel prior to hydrological and geochemical analyses to prevent cross contamination of samples. Ensure that new gloves are worn by all members carrying out the excavation to reduce contamination from human skin.
- Use a soil corer of 1 cm diameter and 20 cm height, and a thin spatula for microbiological sample collection. Clean the corer and the spatula with distilled water, wipe dry with clean wipes, and rinse with 75% ethanol using a spray bottle. Allow corer and spatula to air dry.
- Note the collection time of each sample. Use the corer to core to a depth of 10 cm at each voxel location, and the spatula to empty the soil sample into pre-sterilized plastic bags (Figure 5). Take care to open the bag just prior to depositing the sample. Homogenize the sample pouches by hand.
- Store the sample bag in an ice cooler during sampling, and transfer as soon as possible to the -80 °C freezer.

Figure 5. Microbiology sample collection. A small handheld corer of 20 cm x 1 cm, sterile bags, and spatula is shown here during microbiological sampling. Please click here to download this file.
5. Geochemistry and Hydrology Sample Collection
- Photograph dyed regions in X and Y planes during excavation for depths where the dye is observed. Use a color card to provide reference for the color observed (Figure 6). Ensure proper natural lighting is present to correctly document color intensity.
- Calibrate portable x-ray fluorescence spectrometer (pXRF) daily before starting measurements. For calibration and measurement details, see manufacturer's instructions13 (Figure 7). Briefly, place the instrument on the holder and point the beam window directly to the factory metal bead. Select 'Cal' and wait for 30 sec to allow the calibration to be completed.
- Clean the beam window before taking every measurement. Measure the surface of each voxel in triplicate at three different locations. Place the pXRF instrument on the soil surface and wait for 90 sec to allow the measurement to be completed.
NOTE: X-ray can penetrate through a long distance in the direction of the beam. Therefore, ensure that only a trained personnel handles the equipment and maintains proper safety protocols.
- Clean the beam window before taking every measurement. Measure the surface of each voxel in triplicate at three different locations. Place the pXRF instrument on the soil surface and wait for 90 sec to allow the measurement to be completed.

Figure 6. Color card to follow dye infiltration. Each location with visible dye penetration was photographed with a color card serving as reference. Please click here to download this file.

Figure 7. Portable X-ray Fluorescence Spectrometer. Handheld pXRF positioned on surface of a voxel. Measurements were recorded at three different locations on the surface of each voxel and then averaged.
- Clean metallic cores (height = 3 cm, dia. = 5.7 cm) and polycarbonate cores (height = 6 cm, dia. = 5.7 cm) for bulk densities (BD) and hydraulic conductivity measurements (Ksat) of desired voxels, respectively (Figure 8).
- Vertically insert metal cores and polycarbonate cores (vertical Ksat) into desired voxels taking care not to damage sensors or sensor wires. Do this by gently hammering the cores into the soil, taking care to use a flat surface like block of wood between the core and the hammer in order to minimize disturbance to the soil. Additionally, once the core is halfway into the soil, place a second core on top of the first core. Place the wooden block on top of the second core and gently hammer the block until the first core is embedded in the soil with the core rim still visible.
- Insert cores for horizontal Ksat as the lateral face of the voxel opens up with sequential excavation. Use the wooden block and second core as mentioned in step 5.4 to minimize compaction.
- Take care to ensure that the voxel being sampled is isolated from boundaries and neighboring voxels prior to geochemical sample collection. Use plastic putty knives for this purpose, followed by hand-held trowels to collect soil samples around metal or polypropylene cores into labeled geochemical (GC) sample bags until cores can be easily removed (e.g., Figure 9a, b).

Figure 8. Bulk density and hydraulic conductivity cores. Polypropylene cores (left) were used for collecting vertical and horizontal hydraulic conductivity samples while metal cores (right) were used for collecting bulk density samples.

Figure 9. Voxel demarcation. Plastic putty knives were used to (A) isolate voxel boundaries prior to (B) geochemical, bulk density, and hydraulic conductivity core collection. Please click here to download this file.
- Remove the metallic core, brush off excess material from both ends, and transfer sample from core to a labeled BD sample bag. Weigh each sample bag with sample and record the total weight.
- Remove the polypropylene cores. Cover both sides with red plastic caps and label vertical polypropylene core as "V" and horizontal polypropylene core as "H" followed by the sample ID.
- Collect remaining material from the voxel into the GC sample bag, leaving behind a couple of centimeters of soil at all four sides to prevent cross-contamination with the next voxel.
- Repeat from steps 5.1 to 5.9 for the rest of the voxels in one layer.
- Once all voxels from one layer have been completed, repeat steps from 3.2 to 5.10 for the subsequent layer.
NOTE: Step 5.1 needs to be performed only for the voxels that have visible dye. Refer to Figure 10 to visualize representative diagram of a voxel highlighting all samples collected from each voxel.

Figure 10. Representative voxel. The red dashed line indicates core collected for microbiology sample, the green dashed line indicates horizontal hydraulic conductivity core, the yellow dash line indicates vertical hydraulic conductivity core, the purple dashed indicates bulk density core, and the blue oval boundary indicates remaining sample from the voxel being used for geochemical analysis. Please click here to download this file.
6. Sample Analysis
- Use samples collected for microbiological analyses for molecular (soil microbial DNA extraction)14 and cultured (heterotrophic plate counts)15 analyses. Use extracted DNA for quantitative polymerase chain reactions (qPCR)16, and high-throughput gene sequencing experiments17,18.
- Use samples collected for geochemical analyses to measure a multitude of geochemical properties including pH (US EPA method 150.2), electrical conductivity (EC) (US EPA method 120.1), carbon and nitrogen content (US EPA method 415.3, sequential extraction of elements19, and X-ray diffraction (XRD) and extended X-ray absorption fine structure (EXAFS) spectroscopy as per specifications of Stanford Synchrotron Radiation Laboratory, to investigate mineral transformations.
- Use core samples collected for hydrological analyses for laboratory experiments such as bulk density20 and hydraulic conductivity21.
Wyniki
The dimensions of voxels ensured collection of samples for hydrological, geochemical, and microbiological measurements. The excavation procedure yielded 324 cores for microbiological analysis, 972 pXRF data points, 324 geochemical sample bags, 180 Ksat samples (128 vertical and 52 horizontal), and 311 bulk density samples. Preferential flow of Brilliant Blue dye was also observed to a depth of 30 cm below the surface. A representative set of 81 samples from a single vertical slice of the ...
Dyskusje
Landscape evolution is the cumulative effect of hydrological, geochemical, and biological processes12. These processes control flow and transport of water and elements, and biogeochemical reactions in evolving landscapes. However, capturing the interactions simultaneously requires precisely coordinated experimental design and sampling. Additionally, studying incipient landscape evolution is difficult in natural systems, with limited capabilities to identify "time zero" conditions. Literature reports on...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We thank Ty P.A. Ferré, Till Volkman, Edwin Donker, Mauricio Vera for helping us during the excavation, and Triffon J. Tatarin, Manpreet Sahnan and Edward Hunt for their help in sample analysis. This work was carried out at Biosphere 2, University of Arizona and funded by National Science Foundation grant EAR_1344552 and Honors Research Program of Biosphere 2.
Materiały
| Name | Company | Catalog Number | Comments |
| Measuring tape | Any | Any | Preventing cross-contamination of samples is crucial. Therefore, it is helpful to have multiple putty knives to isolate voxel boundary. |
| Brilliant Blue dye | Waldeck GmBH &Co | B0770 | Rulers can be used to draw grids. The sampling strategy can be modified based on individual experiments. |
| Soil Corer | AMS | 56975 | Any commercially manufactured Brilliant Blue dye can be used. |
| 75% Ethanol | Any | Any | A Nikon D90 camera and 50 mm lens were used for photography. Any high resolution camera and lens can be used for this purpose. |
| Spray Bottle | Any | Any | Use of dye and color card is subjective to individual experiments and/or research questions. |
| Spatula | Any | Any | Gardening gloves may be used if handling of corer becomes tedious. |
| Gloves | Any | Any | Ensure microbiology samples are kept in ice during sampling and frozen as soon as possible. |
| KimWipes | KimTech Science | Any | Water can be used to wash soil corer, prior to sanitizing with ethanol. |
| Sterile Sample bags | Fisher Scientific | Whirl-Pak 4 OZ. 24 OZ | Keep buckets and dustpans handy to facilitate removal of waste soil. |
| Color Card | Any | Any | The original design of miniLEO has various sensors embedded in the lysimeter. Such sensors may or may not be necessary based on the scope of individual experimental design. |
| X-ray Fluoresce Spectrophotmeter | XRF, OLYMPUS | DS-2000 Delta XRF | |
| Polypropylene cores | Any | Any | |
| Metal cores | Any | Any | |
| Caps for polypropylene cores | Any | Any | |
| Hammer | Any | Any | |
| Plastic putty knives | Any | Any | |
| Face masks | Any | Any |
Odniesienia
- Brady, N. C., Weil, R. R. . The nature and properties of soils. , (2008).
- Chorover, J., Kretzschmar, R., Garica-Pichel, F., Sparks, D. L. Soil biogeochemicial processes within the critical zone. Elements. 3, 321-326 (2007).
- Troch, P. A., et al. Catchment coevolution: A useful framework for improving predictions of hydrological change?. Water Resour. Res. 6, 1-20 (2015).
- Sharp, R. P. Landscape evolution (A Review). Proc. Natl. Acad. Sci. U. S. A. 79, 4477-4486 (1982).
- Temme, A., Montgomery, D. R., Bierman, P. R. Predicting the effect of changing climate on landscapes with computer based landscape evolution models. Key Concepts in Geomorphology. , (2013).
- Troch, P. A., et al. Dealing with Landscape Heterogeneity in Watershed Hydrology: A Review of Recent Progress toward New Hydrological Theory. Geogr. Compass. 3, 375-392 (2009).
- Wang, Y., et al. Dissecting the Hydrobiogeochemical Box. in Am. Geophys. Union Fall Meet. , (2015).
- Lin, H., et al. Hydropedology: Synergistic integration of pedology and hydrology. Water Resour. Res. 42 (5), W05301 (2006).
- Band, L. E., et al. Ecohydrological flow networks in the subsurface. Ecohydrology. 7, 1073-1078 (2014).
- Churchman, G. j., Lowe, D. . Handbook of Soil Science Properties and Process. 1, (2012).
- van der Heijden, M. G. A., Bardgett, R. D., van Straalen, N. M. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11 (3), 296-310 (2008).
- Pangle, L. a., et al. The Landscape Evolution Observatory: A large-scale controllable infrastructure to study coupled Earth-surface processes. Geomorphology. 244, 190-203 (2015).
- . . User Manual: Delta Famiy Handheld XRF Analyzers. , (2013).
- Valentìn-Vargas, A., Root, R. A., Neilson, J. W., Chorover, J., Maier, R. M. Environmental factors influencing the structural dynamics of soil microbial communities during assisted phytostabilization of acid-generating mine tailings: A mesocosm experiment. Sci Total Environ. 500-501, 314-324 (2014).
- JoVE Science Education Database. . Essentials of Environmental Microbiology. Culturing and Enumerating Bacteria from Soil Samples. , (2016).
- JoVE Science Education Database. . Essentials of Environmental Microbiology: Quantifying Environmental Microorganisms and Viruses Using qPCR. , (2016).
- Sengupta, A., Dick, W. A. Bacterial community diversity in soil under two tillage practices as determined by pyrosequencing. Microb. Ecol. 70, 853-859 (2015).
- Caporaso, J. G., et al. Correspondence - QIIME allows analysis of high- throughput community sequencing data. Nat. Publ. Gr. 7, 335-336 (2010).
- Hall, G. E. M., Vaive, J. E., Beer, R., Hoashi, M. Selective leaches revisited, with emphasis on the amorphous Fe oxyhydroxide phase extraction. J. Geochemical Explor. 56, 59-78 (1996).
- Grossman, R. B., Reinsch, T. G., Dane, J. H., Topp, G. C. Bulk density and linear extensibility. Methods of Soil Analysis. Part 4-Physical Methods. , 201-228 (2002).
- Reynolds, W. D., Elrick, D. E., Youngs, E. G., Amoozegar, A., Bootlink, H. W. G., Bouma, J., Dane, J. H., Topp, G. C. Saturated and field-saturated water flow parameters. Methods of Soil Analysis, Part 4-Physical Methods. , 802-816 (2002).
- King, G. M. Contributions of atmospheric CO and hydrogen uptake to microbial dynamics on recent Hawaiian volcanic deposits. Appl. Environ. Microbiol. 69 (7), 4067-4075 (2003).
- Meyer, W. S., Barrs, H. D. Roots in irrigated clay soils: Measurement techniques and responses to rootzone conditions. Irrig. Sci. 12 (3), 125-134 (1991).
- Graham, C. B., Woods, R. A., McDonnell, J. J. Hillslope threshold response to rainfall: (1) A field based forensic approach. J. Hydrol. 393 (1-2), 65-76 (2010).
- Anderson, A. E., Weiler, M., Alila, Y., Hudson, R. O. Dye staining and excavation of a lateral preferential flow network. Hydrol. Earth Syst. Sci. Discuss. 5 (2), 1043-1065 (2008).
- Gleeson, T., Paszkowski, D. Perceptions of scale in hydrology: what do you mean by regional scale?. Hydrol. Sci. J. 00, 1-9 (2013).
- Molins, S., Trebotich, D., Steefel, C. I., Shen, C. An investigation of the effect of pore scale flow on average geochemical reaction rates using direct numerical simulation. Water Resour. Res. 48, W03527 (2012).
- Fierer, N., Lennon, J. T. The generation and maintenance of diversity in microbial communities. Am. J. Bot. 98 (3), 439-448 (2011).
- Niu, G. Y., Pasetto, D., Scudeler, C., Paniconi, C., Putti, M., Troch, P. A. Analysis of an extreme rainfall-runoff event at the Landscape Evolution Observatory by means of a three-dimensional physically-based hydrologic model. Hydrol. Earth Syst. Sci. Discuss. 10, 12615-12641 (2013).
- Marteinsson, V., et al. Microbial colonization in diverse surface soil types in Surtsey and diversity analysis of its subsurface microbiota. Biogeosciences. 12, 1191-1203 (2015).
- Orcutt, B. N., Sylvan, J. B., Rogers, D. R., Delaney, J., Lee, R. W., Girguis, P. R. Carbon fixation by basalt-hosted microbial communities. Front. Microbiol. 6, 00904 (2015).
- Wu, L., Jacobson, A. D., Chen, H. C., Hausner, M. Characterization of elemental release during microbe-basalt interactions at T=28°C. Geochim. Cosmochim. Acta. 71, 2224-2239 (2007).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone