Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Assessment of Labile Organic Carbon in Soil Using Sequential Fumigation Incubation Procedures
W tym Artykule
Podsumowanie
Labile organic carbon (LOC) and the potential carbon turnover rate are sensitive indicators of changes in soil nutrient cycling processes. Details are provided for a method based on fumigating and incubating soil in a series of cycles and using the CO2 accumulated during the incubation periods to estimate these parameters.
Streszczenie
Management practices and environmental changes can alter soil nutrient and carbon cycling. Soil labile organic carbon, a readily decomposable C pool, is highly sensitive to disturbance. It is also the primary substrate for soil microorganisms, which is fundamental to nutrient cycling. Due to these attributes, labile organic carbon (LOC) has been identified as an indicator parameter for soil health. Quantifying the turnover rate of LOC also aids in understanding changes in soil nutrient cycling processes. A sequential fumigation incubation method has been developed to estimate soil LOC and potential C turnover rate. The method requires fumigating soil samples and quantifying CO2-C respired during a 10 day incubation period over a series of fumigation-incubation cycles. Labile organic C and potential C turnover rate are then extrapolated from accumulated CO2 with a negative exponential model. Procedures for conducting this method are described.
Wprowadzenie
Due to its vital roles in carbon (C) and nutrient cycling and its sensitivity to soil change, soil LOC is an important parameter to measure as an indicator of soil organic matter quality. Forests and agroecosystems to a large degree depend on the mineralization of nutrients in soil organic matter as a source of nutrients. Management activities can change the pool size and turnover rate of soil organic C, resulting in changes in nutrient supply1. Soil organic C consists of two primary fractions of recalcitrant C, which has turnover rates of several thousand years, and LOC, which has turnover rates from a few weeks to a few years2,3,4. Soil labile C consists of readily decomposable substrates such as microbial biomass C, low-molecular-weight compounds (amino acids, simple carbohydrates) from plant rhizodeposition, and decomposition byproducts and leachates from plant litter1,4,5. Because soil labile C is readily decomposable, it is highly sensitive to management practices and natural phenomena that disturb or alter soil6. Soil labile C serves as the primary energy source for soil microorganisms in the decomposition of organic matter7. As such, LOC impacts nutrient cycling to a greater degree than does stable forms of soil organic C8. Soil microorganisms are also responsible for the majority of heterotrophic respiration that occurs during decomposition of recalcitrant soil organic matter facilitated by the priming effect of LOC9,10,11. This respiration plays a substantial role in global C cycles because soil organic C is approximately double that of atmospheric C11.
As a result of its importance in terrestrial ecosystems, several methods have been developed to estimate soil LOC. These methods can be delineated into three general classifications: physical, chemical, and biochemical. Densitometric separation methods are physical methods that consist of separating soil organic C into heavy or light fractions or into coarse and fine particulate organic C12,13,14,15. Separation methods are relatively easy to perform, but they do not often produce consistent results because these fractions vary with soil type mineral composition, plant material size and density, and soil aggregate consistency13,15. Separation methods also produce only quantitative information about LOC15.
Several chemical methods are available for LOC estimation. Aqueous extraction of organic carbon is relatively easy to perform, and the methods often provide easily reproducible results. However, these extractions do not involve the whole spectrum of available substrates for microorganisms15. Several oxidation methods for chemical fractionation of soil organic C have been developed. Oxidation methods have the advantage of characterizing the quantity and quality of labile organic C, although some methods require work with hazardous chemicals and there is variability among the methods in reproducibility of results15. The acid hydrolysis extraction method is another type of chemical fractionation procedure that can measure the quantity and quality of LOC, but results of this method do not facilitate interpretation of its biological properties13,15.
Biochemical methods for interpretation of soil LOC have been developed. Labile organic C can be measured as CO2 released by microorganisms in respiration assays. These assays provide estimates of true mineralizable organic matter, but typically only the most labile compounds are mineralized during the assays15. Soil microbial biomass C measured by fumigation-incubation16 and fumigation-extraction17 has been used to develop inferences about LOC. However, these procedures provide estimates of C in microbial biomass rather than LOC. Both fumigation procedures include subtraction of values from non-fumigated soil to determine microbial biomass C, but it has been suggested that values obtained without subtraction of non-fumigated soil provide a measure of labile organic fractions of C in addition to microbial biomass18.
The sequential fumigation-incubation (SFI) procedure13 for measuring LOC is a biochemical method adapted from the fumigation-incubation procedure16 for soil microbial biomass C measurement. The SFI method has some advantages relative to other methods of estimating LOC. A conceptual basis for the method is that LOC is the microbially degradable C that governs microbial growth and that LOC is physically accessible and chemically degradable by soil microorganisms. Under field conditions, microbial growth is typically limited by carbon availability, nutrient availability, available pore space, and/or predation. These factors are nearly eliminated by fumigation, creating unimpeded conditions for microbial growth. No nutrients are removed during the incubation period of the method. Over the course of multiple fumigation and incubation cycles, microbial growth becomes limited by C quantity and quality (lability)13. The accumulated CO2 respired during the incubation cycles is used to extrapolate LOC with a simple negative exponential model11,13,19. The potential C turnover rate can also be derived from the slope of the exponential model, so the SFI method has the advantage over most other LOC methods of simultaneously estimating the concentrations and potential turnover rate of LOC11. For other methods, information on the potential turnover rates of LOC can only be ascertained if tracers such as 14C are used13. The SFI method is thus a relatively simple and inexpensive technique for obtaining measurements of both LOC and its potential turnover rates.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Collect Soil to Get Samples Representative of Conditions within the Experimental Area and within Experimental Units20
- Identify any differences in site properties such as slope and soil properties including texture, bulk density, pH, organic horizon depth, and/or nutrient concentrations. Identify any differences in vegetation type within plots. Use known or published estimates of coefficients of variation for site properties to estimate the number of samples required to attain a pre-specified relative error.
- Sample soil using an auger or other collection device in a pattern based on site and experimental unit conditions.
- For homogeneous conditions, use a random sampling pattern within each experimental unit.
- Assign sample points at either completely random locations within the experimental unit or in a zigzag pattern.
- Sample soil at each random point or at points assigned in a zigzag pattern. Brush aside organic matter from the surface of mineral soil before using the auger or other collection device to excavate soil sample.
NOTE: The SFI method was developed using soil horizons from the Oa and below13. Further testing is necessary if horizons above the Oa can be tested using the SFI method. - Combine all samples collected within the experimental unit into a single container and physically mix the individual samples within the container to create a composite sample for each experimental unit.
- For heterogeneous conditions, which are much more common, use a systematic sampling pattern within each experimental unit.
- Sample soils along the transect in the center of each experimental unit such that the distance between sample points within the transect is smaller than the distance needed to represent variability within the experimental units.
- Sample soils along multiple transects within each experimental unit that form a grid pattern in relatively large experimental units or experimental units with multiple sources of variability.
- Combine all samples collected along each transect into a single container and physically mix the individual samples within the container to create a composite sample for each transect.
- For homogeneous conditions, use a random sampling pattern within each experimental unit.
2. Prepare Soil for the SFI Assay
- Place samples in an ice-pack filled cooler immediately after collection in the field.
- Upon arrival at the facility at which samples are to be stored until analysis, place samples in a refrigerator at 4 °C until sample preparation and SFI procedures are conducted.
- Sieve soil samples through a 6.4 mm x 6.4 mm mesh sieve. Clean the mesh with water between each sample to prevent contamination between samples.
- For each sample, measure three 100 g subsamples and place the 100 g subsamples in a 250 ml beaker. Cover each beaker with Parafilm and leave them on a countertop for 10 days at 25 °C.
3. Take Subsamples for Oven-dry Weight Determination
- At the end of the 10 day pre-incubation of soil samples, remove Parafilm from each sample.
- Record the weight of an aluminum weigh boat. Take 1 g of soil from all samples and place in weigh boat.
- Record the weight of the moist soil and weigh boat.
- Place the weigh boats with soil in an oven at 105 °C. After the samples reach a constant weight, which is typically after 48 hr, record weights of the weigh boats and soil.
- Subtract weigh boat weight from the weights taken of the moist soil and dry soil within the weigh boat to get moist and dry soil weight. Derive the dry:moist soil ratio by dividing dry soil weight by the moist soil weight.
4. Fumigate Soil Samples
- Place a damp paper towel in the bottom of at least two (more may be necessary depending on the number of samples) 10.5 L glass vacuum desiccators with porcelain plates.
- For all samples, weigh 30 g of soil into three separate glass vials. Use vials large enough to hold 40 g of soil and narrow enough to fit within a 40 mm opening if the incubation container design described in section 5 is used.
- If using labeling tape to identify each 30 g soil subsample, use pencil because fumigation degrades ink.
- Place two of the three 30 g subsamples for each soil sample into a vacuum desiccator for fumigation and one subsample into a vacuum desiccator that will not conduct fumigation.
- In a 100 ml beaker, place a layer of boiling stones sufficient to cover the bottom of the beaker.
- Pour 50 ml of ethanol-free chloroform (CHCl3) into the 100 ml beaker with a layer of boiling stones. Place the 100 ml beaker with boiling stones and CHCl3 in the center of a desiccator filled with the 30 g soil subsamples. Conduct this step under a fume hood.
- Under a fume hood, use a vacuum to boil the CHCl3 to fumigate two sets of subsamples per soil sample.
- Connect the vacuum to the vacuum desiccator with vacuum tubing. Start the vacuum and watch as CHCl3 begins to boil.
- Allow CHCl3 to boil for 30 sec and disconnect the vacuum tubing from the desiccator to allow air to flow back into the desiccator. This step promotes CHCl3 gas entry into the soil samples. Repeat twice.
- Perform a fourth and final boil of CHCl3, allowing it to boil for 2 min.
- With vacuum still running, close the seal on the vacuum desiccator so that the vacuum within the desiccator is maintained. Turn off the vacuum and disconnect the vacuum tubing from the desiccator.
- Seal the desiccator containing the non-fumigated samples by placing a lid on the desiccator and sealing the vacuum stopper. Place the desiccators (fumigated and non-fumigated) in a darkened area (such as a cabinet) for 24 hr. Do not repeat the vacuum procedures of subsection 4.7 on the desiccator containing non-fumigated samples.
5. Assemble Containers for Soil Sample Incubation
- Push a 15 cm length glass rod through a size 10 rubber stopper with a hole drilled in the center. The rod diameter should be sufficient to fit through the hole snugly.
- Label 0.5 L translucent wide mouth polypropylene bottles with identification that corresponds to the fumigated and non-fumigated subsample identification.
6. Evacuate Chloroform from Desiccators Under a Fume Hood
- Open the stopper on a vacuum desiccator to allow airflow into the desiccator. Remove the lid from the desiccator, and take the samples and the damp towel out of desiccator.
- Use a vacuum to evacuate CHCl3 gas from soil samples.
- Place the lid on the desiccator. Connect the desiccator to a vacuum with vacuum tubing.
- Turn on the vacuum pump and allow pump to run for five minutes. Disconnect the vacuum tubing from the desiccator to allow airflow into the desiccator.
- Repeat step 6.3.2 four times.
7. Move Each Soil Subsample into an Incubation Container (Figure 1) to Conduct a 10 Day Incubation
- Pipette 1 ml of deionized water into the incubation container. Connect an empty glass vial to the glass rod extending from the size 10 stopper using a rubber band. The open end of the glass vial should face the base of the stopper. The glass vial should be of a size sufficient to hold up to 40 ml of fluid.
- Place a vial containing the 30 g soil subsample into the incubation container.
- Add 1 g of non-fumigated soil from the original soil sample to each of its corresponding subsamples (fumigated and non-fumigated) as inoculum.
- Pipette 1 ml of 2 M NaOH into the glass vial connected to the stopper/glass rod. Push the stopper/glass rod onto the top of the incubation container. Cover the top of the incubation container with Parafilm.
- Create an incubation container that contains no soil. Assemble three to five no-soil incubation containers.
NOTE: The acid used to titrate samples of the no-soil container is essential to the determination of CO2 mineralization during the incubation period, which is described below in subsection 9.3. As such, multiple no-soil containers are created as a safeguard against incorrect handling or titration of a no-soil incubation container that would create an error in CO2 mineralization calculation for all samples. The acid used to titrate samples from the no-soil containers should be close in values; a highly dissimilar acid value among the no-soil container samples is likely the result of incorrect sample handling or titration.- Follow the procedures of section 5 to assemble incubation containers.
- Follow the procedures of 7.1 and 7.4.
- Place all incubation containers in a darkened storage area at 25 °C. Leave all incubation containers in the storage area for 10 days.
8. Perform Titration on Each Subsample to Quantify CO2 Produced by Microbial Respiration during the Incubation Period
- Remove the glass vial containing the 2 M NaOH from the incubation container.
- Pipette 2 ml of 1 M BaCl2 into the glass vial containing 2 M NaOH.
- Add one drop of phenolphthalein (C20H14O4) from a pipette or medicine dropper into the glass vial containing the mixture of BaCl2 and NaOH. Place a magnetic stir bar in the glass vial and place the glass vial on a stir plate.
- With the stir plate activated, slowly add 0.1 N HCl with a burette until the red coloration of the mixture in the glass vial turns clear.
- Record the amount of HCl required to change the coloration of the mixture in the glass vial.
9. Determine Microbial Biomass C from Data Collected during the First Fumigation-incubation Cycle16,21,22
- Determine the dry weight of soil in each subsample by multiplying its moist weight by the dry:moist weight ratio obtained in step 3.8.
- Determine the average amount of HCl used to titrate the no-soil incubation containers.
- Calculate CO2 mineralized during the 10-day incubation using the formula:

where CO2 = CO2 mineralized during the 10-day incubation
NS = Acid used to titrate samples in no-soil incubation container
S = Acid used to titrate samples that contained soil in the incubation container
M = molarity of the HCl
E = 6, the equivalent weight
W = dry weight of soil contained in the incubation container - Calculate microbial biomass C using the formula:
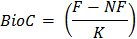
where BioC = microbial biomass C
F = CO2 mineralized from soil subsamples that were fumigated
NF = CO2 mineralized from soil subsamples that were non-fumigated
K = fraction of microbial biomass C mineralized to CO2- Determine the value for K by either direct measurement of 14C mineralization in preliminary tests with the soil or published values22. A value of 0.45 is commonly used for K for this assay23.
- Perform sequential fumigation and incubation cycles by repeating sections 4-8 seven times for the soil subsamples that were fumigated in the first fumigation-incubation cycle.
10. Determine Labile C and Potential C Turnover Rate Using CO2 Mineralized over the Course of the Eight Fumigation and Incubation Cycles
- Use the following formula to determine a correction factor for the soil inoculum added to samples after each fumigation:
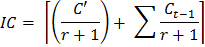
Where IC = Correction factor for inoculum
C' = Amount of CO2 from the non-fumigated subsample during the first 10-day incubation
r = Weight ratio of inoculum soil to fumigated soil in the first fumigation incubation cycle
Ct = incubation cycle (1, 2 … 8), such that Ct-1 = 0 when t = 1 - Use the following formula to estimate the CO2 released during each incubation for each subsample:
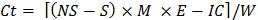
where Ct = CO2 released during incubation
NS = Acid used to titrate samples in no-soil incubation container
S = Acid used to titrate samples that contained soil in the incubation container
IC = Correction factor for inoculum (determined in step 10.1)
E = 6, the equivalent weight
W = dry weight of soil contained in the incubation container - Derive labile organic C using non-linear regression.
- Organize a spreadsheet that includes for each sample identifiers for the sample, incubation cycle number (1, 2 …8), and CO2 released during the incubation (derived in step 10.2).
- Using software capable of non-linear regression, fit the following model to the dataset:

where Csum = the sum of CO2 released during the eight incubation cycles
LOC = soil labile organic C
k = potential turnover time
t = incubation cycle (1, 2 …8)
- Convert potential turnover time from step 10.3.2 into days by multiplying the inverse of k by 10 due to the 10 day incubation cycle.
Access restricted. Please log in or start a trial to view this content.
Wyniki
The SFI method has been used as described in this paper in a series of experiments conducted in the southeastern United States24,25,26,27. Together, these experiments encompassed a variety of vegetation types, including loblolly pine (Pinus taeda L.), switchgrass (Panicum virgatum L.), cottonwood (Populus deltoides Bartram ex Marsh.), and soybean (Glycine max L. Merr.). The method was sensitive at determining differences in LOC and/or potenti...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
The SFI method is an effective protocol for detecting differences in soil LOC and potential C turnover rates over a range of management practices (such as fertilization, tillage, vegetation control, and harvest practices) and soil conditions. Soil LOC content and C turnover rate can be used to understand alterations of nutrient cycles. The SFI method also provides measurement of microbial biomass C from the first fumigation-incubation event. The ability to measure soil LOC, C turnover, and microbial biomass C concurrentl...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors gratefully acknowledge Michelle Gonzales, Kenny Kidd, Brad Osbon, and all other personnel that conducted the laboratory procedures for these data. The authors are thankful for assistance from Andrew Scott in developing software coding to conduct model-fitting procedures. The authors also appreciate the funding from the U.S. Department of Agriculture National Institute of Food and Agriculture, Sustainable Agriculture and Research & Education, Sun Grant South Central region, and the National Council of Air and Stream Improvement that made possible the studies from which representative results provided in this paper were drawn.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Soil auger sampling kit | JMC | PN039 | Several other manufacturers of punch augers are available |
| Parafilm | Curwood | PM999 | |
| Aluminum weighing boats | Fisherbrand | 08-732-103 | |
| General purpose drying oven | Fisher Scientific | 15-103-0511 | Many other manufacturers of general purpose laboratory ovens are available |
| 10.5 L vacuum desiccator | Corning | 3121-250 | |
| Glass scintillation vial | Wheaton | 968560 | |
| Glass threaded vials, 41 ml | Fisherbrand | 03-339-21N | |
| Chloroform, stabilized with amylenes | Sigma-Aldrich | 67-66-3 | |
| Boiling chips | Fisher Scientific | S25201 | |
| Glass rod | Fisherbrand | S63449 | |
| Size 10 rubber stopper | Fisherbrand | 14-130P | Rubber stoppers can be purchased as solid and drilled in center to install glass rod or bought with a hole to insert glass rod |
| Wide-mouth PPCO bottle, 0.5 L | ThermoScientific | 3121050016 | |
| Sodium hydroxide, reagent grade | Sigma-Aldrich | S5881 | |
| Barium chloride | Sigma-Aldrich | 202738 | |
| Phenolphthalein indicator | Fisher Scientific | S25466 | |
| Hydrochloric acid solution, 0.1 N | Fisher Scientific | SA54-4 |
Odniesienia
- Blair, G., et al. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 46, 1459-1466 (1995).
- Schimel, D. S., et al. Soil organic matter dynamics in paired rangeland and cropland toposequences in North Dakota. Geoderma. 36, 201-214 (1985).
- Parton, W. J., et al. Analysis of factors controlling soil organic matter levels in great-plains grasslands. Soil Sci. Soc. Am. J. 51, 1173-1179 (1987).
- Wu, H., et al. Labile organic C and N mineralization of soil aggregate size classes in semiarid grasslands as affected by grazing management. Biol. Fertil. Soils. 48, 305-313 (2011).
- Jones, D. L., et al. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 163, 459-480 (2004).
- Harrison, K. G., et al. The effect of changing land use of soil radiocarbon. Science. 262, 725-726 (1993).
- Jinbo, Z., et al. Land use effects on the distribution of labile organic carbon fractions through soil profiles. Soil Sci Soc. Am. J. 70, 660-667 (2006).
- Whalen, J. K., et al. Carbon and nitrogen mineralization from light- and heavy-fraction additions to soil. Soil Biol Biochem. 32, 1345-1352 (2000).
- Gregorich, E. G., et al. Towards a minimum data set to assess soil organic matter quality in agricultural soils. Can. J. Soil Sci. 74, 367-385 (1994).
- Hamer, U., et al. Priming effects in different soil types induced by fructose, alanine, oxalic acid and catechol additions. Soil Biol. Biochem. 37, 445-454 (2005).
- Feng, W., et al. Shifting sources of soil labile organic carbon after termination of plant carbon inputs in a subtropical moist forest of southwest China. Ecol. Res. 26, 437-444 (2011).
- Tisdall, J. M. Formation of soil aggregates and accumulation of soil organic matter. Structure and Organic Matter Storage in Agricultural Soils. Carter, M. R., Stewart, B. A. , Lewis Publishers. 57-96 (1996).
- Zou, X. M., et al. Estimating soil labile organic carbon and potential turnover rates using a sequential fumigation-incubation procedure. Soil Biol. Biochem. 37, 1923-1928 (2005).
- Cambardella, C. A., Elliott, E. T. Particulate soil organic matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 56, 777-783 (1992).
- Strosser, E. Methods for determination of labile soil organic matter: an overview. J. Agrobiol. 27, 49-60 (2010).
- Jenkinson, D. A., Powlson, D. S. The effects of biocidal treatment on metabolism in soil V: a method for measuring soil biomass. Soil Biol. Biochem. 8, 209-213 (1976).
- Vance, E. D., et al. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703-707 (1987).
- De-Polli, H., et al. Chloroform fumigation-extraction labile C pool (microbial biomass C "plus") shows high correlation to microbial biomass C in Argentinian and Brazilian soils. Cienc. Suelo. 25, 15-22 (2007).
- Olson, J. S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology. 44, 322-331 (1963).
- Pennock, D., et al. Chapter 1, Unit 1, Soil sampling designs. Soil Sampling and Methods of Analysis. Carter, M. R., Gregorich, E. G. , CRC Press, Taylor & Francis Group, LLC. (2008).
- Luizao, R. C. C., et al. Seasonal variation of soil microbial biomass: the effects of clearfelling a tropical rainforest and establishment of pasture in the central Amazon. Soil Biol. Biochem. 24, 805-813 (1992).
- Horwath, W. R., Paul, E. A., et al. Microbial biomass. Methods of soil analysis part 2: microbiological and biochemical properties. Weaver, R. W. , Soil Science Society of America, Inc. 753-773 (1994).
- Jenkinson, D. S., Ladd, J. N. Microbial biomass in soil: measurement and turnover. Soil Biochemistry. Paul, E. A., Ladd, J. N. , Marcel Dekker. 415-471 (1981).
- Blazier, M. A., et al. Poultry litter fertilization impacts on soil, plant, and water characteristics in loblolly pine (Pinus taeda L.) plantations and silvopastures in the mid-South USA. Principles, application, and assessment in soil science. Gungor, E. B. O. , InTech, Inc. 43-74 (2011).
- Blazier, M. A., et al. Straw harvesting, fertilization, and fertilizer type alter soil biophysical properties in a loblolly pine plantation in the mid-South USA. Biol. Fertil. Soils. 45, 145-153 (2008).
- Blazier, M. A., et al. Loblolly pine age and density affects switchgrass growth and soil carbon in an agroforestry system. For. Sci. 58, 485-496 (2012).
- Blazier, M. A., et al. Nitrogen and carbon of switchgrass, loblolly pine, and cottonwood biofuel production systems in the Southeast United States. For. Sci. 61, 522-534 (2015).
- Zhang, M., et al. Decomposition differences of labile carbon from litter to soil in a tropical rain forest and rubber plantation of Xishuagbanna, southwest China. Eur. J. Soil Biol. 55, 55-61 (2013).
- Nelson, D. W., Sommers, L. E. Total carbon, organic carbon, and organic matter. Methods of soil analysis. Part 3: chemical methods. Sparks, D., et al. , Soil Science Society of America, Inc. 961-1090 (1996).
- Huang, L., et al. Correlation among soil microorganisms, soil enzyme activities, and removal rates of pollutants in three constructed wetlands purifying micro-polluted river water. Soil Biol. Biochem. 70, 221-228 (2012).
- Kong, L., et al. Enzyme and root activities in surface-flow constructed wetlands. Chemosphere. 76, 601-608 (2009).
- Cui, L., et al. Evaluation of nutrient removal efficiency and microbial enzyme activity in a baffled subsurface-flow constructed wetland system. Bioresour. Technol. 146, 656-662 (2013).
- Jenkinson, D. S. Determination of microbial biomass carbon and nitrogen in soil. Advances in nitrogen cycling in agricultural ecosystems. Wilson, J. R. , CAB International. 368-386 (1988).
- Sparling, G. P., et al. Interference from plant roots in the estimation of soil microbial ATP, C, N, and P. Soil Biol. Biochem. 17, 275-278 (1985).
- Christie, P., Beatte, J. A. M. Significance of sample size in measurement of soil microbial biomass by the chloroform fumigation-incubation method. Soil Biol. Biochem. 19, 149-152 (1987).
- McLaughlin, K. K., Hobbie, S. E. Comparison of labile soil organic matter fractionation techniques. Soil Sci. Soc. Am. J. 68, 1616-1625 (2004).
- Xia, X., et al. Variation of soil labile organic carbon pools along an elevational gradient in the Wuyi Mountains, China. J. Resour. Ecol. 1, 368-374 (2010).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone