Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
An IL-8 Transiently Transgenized Mouse Model for the In Vivo Long-term Monitoring of Inflammatory Responses
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
The method described here allows for the visualization of IL-8 promoter-dependent inflammation activation in the lungs of mice through non-invasive bioluminescence imaging (BLI). The same animal can be subjected to BLI multiple times for up to two months from the time of delivery of the luciferase reporter construct.
Streszczenie
Airway inflammation is often associated with bacterial infections and represents a major determinant of lung disease. The in vivo determination of the pro-inflammatory capabilities of various factors is challenging and requires terminal procedures, such as bronchoalveolar lavage and the removal of lungs for in situ analysis, precluding longitudinal visualization in the same mouse. Here, lung inflammation is induced through the intratracheal instillation of Pseudomonas aeruginosa culture supernatant (SN) in transiently transgenized mice expressing the luciferase reporter gene under the control of a heterologous IL-8 bovine promoter. Luciferase expression in the lung is monitored by in vivo bioluminescent image (BLI) analysis over a 2.5- to 48-h timeframe following the instillation. The procedure can be repeated multiple times within 2 - 3 months, thus permitting the evaluation of the inflammatory response in the same mice without the need to terminate the animals. This approach permits the monitoring of pro- and anti-inflammatory factors acting in the lung in real time and appears suitable for functional and pharmacological studies.
Wprowadzenie
Chronic lung diseases, such as asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and bronchiectasis, are characterized by airway inflammation. Airway inflammation is characterized by edema, cellular infiltration, T lymphocyte and mast cell activation, increased airway secretions, and excessive collagen deposition. CF is a multisystem disorder, and its major cause of mortality and morbidity is lung bacterial infection with increasing pulmonary exacerbation. The decline in lung function predicts a significantly poorer outcome1,2,3,4.
The inflammation state of the respiratory tract is usually observed through the evaluation of immunological markers recruited during the inflammatory process in material derived from the lower and upper airways, such as sputum, which provides variable results. Bronchoscopies are also performed5. Murine models are valuable tools for investigating the pathogenesis and evolution of diseases characterized by airway inflammation and for which effective treatments or cures have not yet been identified. Animal models of lung infection and inflammation have been used to study asthma and host-pathogen interactions, including the role of chemicals that simulate human conditions (e.g., cigarette smoke exposure, LPS, elastase, ovalbumin, poly I:C, etc., as well as combinations of the above)6. The measurement of inflammation-related parameters requires the sacrifice of the animals, as invasive approaches are required to measure factors such as bacterial load, cytokines in the lungs, and collected bronchoalveolar lavage (BAL) fluid. Also, histological examinations are often required. The possibility of obtaining information on the inflammatory response kinetics requires the use of numerous mice. Therefore, a technique that would allow for obtaining such information without the need to sacrifice the animals is valuable on technical, ethical, economical, and operational bases.
IL-8 is an essential player in the inflammation process, recruiting leukocytes to the inflamed tissue. It represents a molecular read-out for the study of inflammatory pathway activation. MIP-2 and KC may be functional homologs of human IL-8 in mice. Mice express only one potential IL-8 receptor, a homolog of human CXCR27,8, but they are capable of modulating a heterologous IL-8 gene promoter that drives a reporter gene. A lung inflammation murine model has recently been developed after the observation that a bovine IL-8 promoter/luciferase reporter construct can be transactivated in mice. This feature allows for the utilization of bioluminescence imaging (BLI) to monitor the inflammatory response in living animals9.
This model has been adapted to study inflammation triggered by bacterial exoproducts (e.g., LPS or products released by bacterial strains) or TNFalpha10,11. The drug discovery process is focused on the development and optimization of old and new anti-inflammatory molecules that can treat lung diseases, such as CF, asthma, and COPD. These new chemical entities must be quickly and conveniently tested in animal models that can be linked to specific clinical phenotypes in order to facilitate the design of smart clinical trials.
Access restricted. Please log in or start a trial to view this content.
Protokół
All animal experiments described were approved by the intramural animal-welfare committee for animal experimentation by the Interdepartmental Centre of Experimental Research Service at the University of Verona and comply with the European Directive 2010/63 UE, Italian D.Lgs 26/2014 and the revised "Guide for the Care and Use of Laboratory Animals," Washington, D.C.: National Academy Press, 1996. This protocol and experimentation were approved by the National Institutes of Health (n 273/15). Animals had free access to standard rodent chow and softened tap water and were acclimatized for at least 5 days to the local vivarium conditions (room temperature: 20 - 24 °C; relative humidity: 40 - 70%; light-dark cycle: 12 h) before any treatment.
1. In Vivo Gene Delivery
- Use a laminar flow hood to prepare the in vivo delivery reagent/nucleic acid complexes.
- Define the experimental protocol and parameters following the manufacturer's in vivo instructions.
- Use a total injection volume of 200 µL of complexes per mouse.
- Start with 40 µg of DNA suspended in endotoxin-free water; the optimization range can reach 60 µg.
NOTE: The final concentration of nucleic acid in the injection volume must not exceed 0.5 µg/µL, according to the manufacturer's instructions. - Use an N/P ratio of 6 - 8 (0.12 - 0.16 µL of delivery reagent per µg of nucleic acid). Calculate the corresponding volume of delivery reagent.
NOTE: The complexes should be cationic for effective cell entry. The N/P ratio is defined as the number of nitrogen residues (N) on the in vivo delivery reagent per nucleic acid phosphate (P) and represents the measure of the ionic balance within the complexes.
- Dilute the calculated amount (see step 1.2.1) of nucleic acid in 5% glucose (final concentration) using 10% glucose stock solution (provided) and sterile water. Make sure that the dilution volume is half the final injection volume. Vortex gently or mix by pipetting up and down.
- Dilute the calculated amount (see step 1.2.3) of delivery reagent into half the injection volume of 5% glucose (final concentration) using the 10% glucose stock solution (provided) and sterile water. Vortex gently and spin at 13,000 x g for 15 s.
- Add the above diluted delivery reagents to the diluted nucleic acid all at once. Mix them by gentle vortexing and spin down at 13,000 x g for 15 s.
- Incubate the mixture from step 1.5 for 15 min at room temperature.
NOTE: From this point on, the complexes are stable for 4 h at room temperature and for up to 7 days when stored at 4 °C. - Perform tail-vein injections using complexes equilibrated at room temperature. Place the mouse tail into warm water (50 - 53 °C) for 30 s to allow for vein dilation.
- Place the mouse inside the restraining device. Insert a 27- to 30-gauge needle in the tail vein at a 20 - 30° angle and slowly inject 200 µL. Upon completion, remove the needle and apply pressure to the injection site.
NOTE: A slight bulge in the tail during the injection indicates incorrect positioning. If this occurs, remove the needle and repeat the process proximal to the previous site.
- Place the mouse inside the restraining device. Insert a 27- to 30-gauge needle in the tail vein at a 20 - 30° angle and slowly inject 200 µL. Upon completion, remove the needle and apply pressure to the injection site.
- Visualize gene expression by performing in vivo BLI (see step 2) at 24 and 48 h after the intravenous injection.
2. In Vivo BLI
NOTE: Beforehand, prepare a fresh stock solution of 15 mg/mL D-luciferin in DPBS, filter-sterilize it using a 0.22-µm filtering unit, and store it at -20 °C.
- Place the mice into a clear plexiglass anesthesia chamber. Make sure that the isoflurane chamber is full. When ready to anesthetize the animals, ensure that the pump (left) and chamber (right) switches are on. Turn the isoflurane dial to 2.5% for induction and 2% for maintenance. Animals inside the IVIS chamber are kept under 2.5% isofluorane anesthesia during image acquisition.
- After the mice are fully anesthetized, inject 10 mL/kg bodyweight of the D-luciferin solution by an intraperitoneal route 15 min before imaging.
NOTE: A kinetic study on D-luciferin should be performed to determine the time of the signal peak after the administration of D-luciferin. - Open the in vivo imaging system and prepare the imaging chamber by lining it with a piece of black cardstock (discard upon completion). Place the nose cones as needed for correct anesthesia (use one cone per mouse).
NOTE: The tube that supplies the anesthesia to the instrument is split so that the same concentration of anesthesia is plumbed to the anesthesia manifolds located inside the imaging system. - Transfer the mice (up to 5) from the box to the nose cones attached to the manifold in the imaging system and close the door. Image acquisition lasts 5 min.
- Acquire a BLI using the manufacturer's software, as follows.
- Initialize the software. In the in vivo imaging system acquisition control panel, put a check mark next to Luminescent. Confirm that the Excitation Filter setting is Block and the Emission Filter setting is Open.
- Click the arrows: for the Luminescent Imaging Mode, select a 5-min exposure time, Binning 8, and F/Stop 1; for the Photograph Imaging Mode, select Binning 4 and F/stop 8.
- From the Field of View dropdown list, select D, 19 cm, and a Subject Height of 1.5 cm. Click Acquire when ready to acquire the image.
- When the image acquisition is complete, place the mice back into their cages.
- Quantify the photons emitted from specific regions using the manufacturer's software.
- Click ROI Tools in the tool palette. In the ROI Tools, select Measurement ROI from the Type dropdown list.
- Click on the Square icon and draw a squared ROI with the proper dimensions to cover the thorax of one animal. Copy and paste the ROI for each animal to obtain ROIs with the same dimensions. In the ROI Tools panel, click on Measure ROI to obtain the measurements of the total intensity in the ROIs.
- Observe the ROI Measurements data for all ROIs created in the images or sequences during a session (one ROI per row). Click Export and select the folder where the file will be saved.
3. Mouse Challenge with Pro-Inflammatory Stimuli
NOTE: Prior to the mouse challenge with pro-inflammatory stimuli, check the baseline activation by in vivo BLI (see step 2). At least 7 days must pass between in vivo gene delivery and mouse challenge to allow the mild and transient inflammation to disappear.
- Prepare the equipment for intratracheal instillation (refer to Figure 1 and Figure 2).
- Connect the 5 ml disposable syringes with the spring (C, E), a 100 µL syringe (B), and a disposable gauge (D) to the 3-way stopcock (A). Place the system on the support (H). Place the system on the support (H).
- Connect the PE190 micro medical tubing (F) to the disposable gauge (D) and to the pen century (G).
- Fill the 5-mL syringe with 800 µL of air and turn the 3-way stopcock.
NOTE: Fill the tube with Pseudomonas aeruginosa culture supernatant by aspirating 50 µl in the 100 µl syringe.

Figure 1: Schematic Representation of a Single Component of the Intratracheal Device.
(A) 3-way stopcock, (B) 100-µL Hamilton syringe, (C) disposable 5-mL syringe, (D) disposable gauge, (E) disposable 5-mL syringe with spring, (F) PE190 micro medical tubing, (G) pen century, and (H) support. Please click here to view a larger version of this figure.
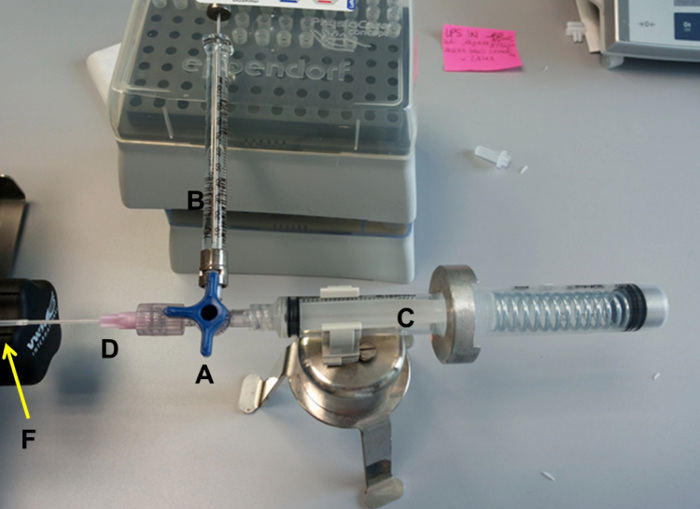
Figure 2: Representation of the Assembled Intratracheal Device.
The identifications are the same as in Figure 1. Please click here to view a larger version of this figure.
- Anesthetize the mice using an isoflurane vaporizer chamber set at 2.5% isoflurane mixed with oxygen.
- Monitor the animal to evaluate the effects of the anesthetic after 3 - 5 min.
NOTE: To confirm that the mouse is fully anesthetized, monitor the following signs carefully: slowed breathing rate should slow down, lack of arm stretching when picked up by the neck, and a lack of response when the hind limbs are stimulated. Wait for a few additional minutes and check again before proceeding to the next step if these criteria are not met. - Place the anesthetized mouse on the plexiglass intubation platform, hanging it by its incisors, which are placed on the wire.
- Turn on the laryngoscope with the left hand (for right-handed researchers) and grab a pair of blunt-ended forceps. Use the tip of laryngoscope and the forceps to gently pry open the mouth.
- Pull the tongue out and hold it to the side using the forceps. Guide the laryngoscope blade towards the back of the mouth. Keep the laryngoscope pressed down very gently at a 90° angle until the opening of the trachea is visible. Hold the laryngoscope in place.
- Using the other hand, take the delivery tube connected to the end of the PE tubing and insert it into the trachea. Rotate the three-way valve to deliver the inoculum. Pull the tube out of the trachea as soon as possible. Hold the mouse upright for a few seconds to the allow inoculum to be inhaled into the lungs.
NOTE: Mice will suffocate and die if the trachea is blocked for too long. - Remove the mouse from the platform. The recovery time may vary based on strains; monitor the mouse diligently, ensuring that the animal is fully awake within 30 min after the procedure.
- Intraperitoneally inject 150 mg/kg D-luciferin and image the lungs using an in vivo imaging system 4, 24, and 48 h after the intratracheal instillation of the stimuli. Quantify the photons emitted from specific regions using the manufacturer's software.
Access restricted. Please log in or start a trial to view this content.
Wyniki
The bIL-8-Luc transient transgenic mouse model was used for the in vivo monitoring of lung inflammation in mice challenged with concentrated bacterial supernatant (30x) containing secreted virulence factors. The induced inflammatory response was detectable by in vivo imaging as an increase in the BLI signal. Pro-inflammatory activity was clearly detectable 2.5 h post-instillation, although the BLI signal reached the highest peak between 5 and 24 h and was still detectabl...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
In a previous work11, a contrast between bIL-8-Luc-dependent BLI and BAL markers was shown. It relied on the differential degree of sensitivity within mouse strains12. For this reason, the first application of the bIL-8-Luc model to a different mouse strain requires an initial study of the inflammatory response, both in terms of BLI and more standardized inflammatory markers.
Mice transfection causes mild lung inflammation and the activation of b...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by the Italian Cystic Fibrosis Foundation Project FFC#18/2013, FFC#29/2015 and by the Italian Cystic Fibrosis League through the Veneto Branch—Associazione Veneta Lotta contro la Fibrosi Cistica Onlus.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| FMT 2500 Fluorescence Tomography System | Perkin Elmer Inc. | Experimental Builder | |
| IVIS Lumina serie II Pre-clinical In Vivo; Imaging System | Perkin Elmer Inc. | Experimental Builder | |
| MMPsense 750 FAST | Perkin Elmer Inc. | NEV10001EX | Protect from light, store the probe at 4 °C |
| Female inbred BalbC | Harlan Laboratories Italy | Prior to use, animals were acclimatized for at least 5 days to the local vivarium conditions | |
| bIL-8-Luc plasmid | Department of Medical Veterinary Science, University of Parma, Italy | Store the plasmid at -20 °C | |
| pGL3basic vector | Promega | E1751 | Store the vector at -20 °C |
| JetPEI DNA transfection reagent | Polyplus transfection | 201B-001G | The DNA and JetPEI mix was formulated with a final N/P ratio of 7 |
| D-luciferin potassium salt 1 g | Perkin Elmer Inc. | 122796 | Protect from light, store at -20 °C |
| Living Image software | Caliper Life Sciences, | Experimental Builder | |
| Isoflurane | ESTEVE spa | 571329.8 | Do not inhale |
| Bio-Plex Cytokine Assay Kit | Bio-Rad Laboratories | M60-009RDPD | Store the unopened kit at 4 °C |
| Automated cell counter | Dasit XT 1800J | Experimental Builder | |
| Penn-century model DP-4M Dry power insufflator | Penn-century | DPM-EXT | |
| Gas anesthesia system XGI-8 | Perkin Elmer Inc. | Experimental Builder | |
| PE190 micro medical tubing | 2biological instruments snc | BB31695-PE/8 | |
| Syringe without needle 5 mL | Terumo | SS*05SE1 | Cut the boards of the piston by a scissors |
| Hamilton 0,10 mL (model 1710) | Gastight | 81022 | |
| Discofix 3-way Stopcock | Braun | 4095111 | |
| Syringe with needle 1 mL | Pic solution | 3,071,260,300,320 | Use without needle |
| Plastic feeding tubes 18ga x 50 mm | 2biological instruments snc | FTP-18-50 | Cut obliquely the tip |
Odniesienia
- Barnes, P. J. Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J Allergy Clin Immunol. 136 (3), 531-545 (2015).
- Cohen-Cymberknoh, M., Kerem, E., Ferkol, T., Elizur, A. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax. 68 (12), 1157-1162 (2013).
- Dhooghe, B., Noel, S., Huaux, F., Leal, T. Lung inflammation in cystic fibrosis: pathogenesis and novel therapies. Clin Biochem. 47 (7-8), 539-546 (2014).
- Durham, A. L., Caramori, G., Chung, K. F., Adcock, I. M. Targeted anti-inflammatory therapeutics in asthma and chronic obstructive lung disease. Transl Res. 167 (1), 192-203 (2015).
- Sagel, S. D. Noninvasive biomarkers of airway inflammation in cystic fibrosis. Curr Opin Pulm Med. 9 (6), 516-521 (2003).
- Starkey, M. R., et al. Murine models of infectious exacerbations of airway inflammation. Curr Opin Pharmacol. 13 (3), 337-344 (2013).
- Cacalano, G., et al. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 265 (5172), 682-684 (1994).
- Simonet, W. S., et al. Long-term impaired neutrophil migration in mice overexpressing human interleukin-8. J Clin Invest. 94 (3), 1310-1319 (1994).
- Stellari, F. F., et al. In vivo imaging of transiently transgenized mice with a bovine interleukin 8 (CXCL8) promoter/luciferase reporter construct. PLoS One. 7 (6), e39716(2012).
- Stellari, F., et al. In vivo imaging of the lung inflammatory response to Pseudomonas aeruginosa and its modulation by azithromycin. J Transl Med. 13, 251(2015).
- Stellari, F., et al. In vivo monitoring of lung inflammation in CFTR-deficient mice. J Transl Med. 14 (1), 226(2016).
- De Simone, M., et al. Host genetic background influences the response to the opportunistic Pseudomonas aeruginosa infection altering cell-mediated immunity and bacterial replication. PLoS One. 9 (9), e106873(2014).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone