Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Using Retinal Imaging to Study Dementia
W tym Artykule
Podsumowanie
The retina shares prominent similarities with the brain and thus represents a unique window to study vasculature and neuronal structure in the brain non-invasively. This protocol describes a method to study dementia using retinal imaging techniques. This method can potentially aid in diagnosis and risk assessment of dementia.
Streszczenie
The retina offers a unique “window” to study pathophysiological processes of dementia in the brain, as it is an extension of the central nervous system (CNS) and shares prominent similarities with the brain in terms of embryological origin, anatomical features and physiological properties. The vascular and neuronal structure in the retina can now be visualized easily and non-invasively using retinal imaging techniques, including fundus photography and optical coherence tomography (OCT), and quantified semi-automatically using computer-assisted analysis programs. Studying the associations between vascular and neuronal changes in the retina and dementia could improve our understanding of dementia and, potentially, aid in diagnosis and risk assessment. This protocol aims to describe a method of quantifying and analyzing retinal vasculature and neuronal structure, which are potentially associated with dementia. This protocol also provides examples of retinal changes in subjects with dementia, and discusses technical issues and current limitations of retinal imaging.
Wprowadzenie
Owing to increases in life expectancy, dementia has become a major medical problem, contributing to significant social and economic health burden globally1,2,3,4,5. Today, a person in the United States develops Alzheimer’s Disease (AD), the most common form of dementia, every 66 s6. It has been estimated that by the year 2050, 115 million people will be affected by AD7.
The retina offers a unique “window” to study dementia due to its similar anatomical and physiological properties with the brain. In terms of vasculature, the retinal arterioles and venules, measuring 100 to 300 µm in diameter, share similar features with cerebral small vessels, such as end arterioles without anastomoses, barrier function, and auto-regulation8,9. In terms of neuronal structure, retinal ganglionic cells (RGCs) share typical properties with neurons in the central nervous system (CNS)10. The RGCs are prominently connected with the brain as they form the optic nerve and project visual signals from the retina to the lateral geniculate nuclei and the superior colliculus. The optic nerve, similar to many neuronal fibers in the CNS, is myelinated by oligodendrocytes and is ensheathed in meningeal layers. Notably, an insult to the optic nerve can result in similar responses observed in other CNS axons, such as retrograde and anterograde degeneration of the axon, scar formation, myelin destruction, secondary degeneration, and an abnormal level of neurotrophic factors and neurotransmitters11,12,13,14. The appearance of visual symptoms in some AD patients may also be explained by the robust associations between the retina and the brain15,16. As a result, it has been suggested that the retina may reflect the pathological processes of dementia in the brain and retinal imaging can be used to study dementia.
The retinal vasculature and neuronal structure can now be visualized non-invasively using retinal imaging techniques. For instance, retinal fundus photographs can be captured using fundus cameras, and characteristics of the retinal vasculature (e.g., vessel caliber, tortuosity, and fractal dimension) can then be quantified using computer-assisted analysis programs. In addition, parameters of the retinal neuronal structure (such as the thickness of ganglion cell-inner plexiform layer [GC-IPL] and retinal nerve fiber layer [RNFL]) can also be measured using optic coherence tomography (OCT) and quantified using the built-in analysis algorithms.
In view of the importance of retinal imaging to studying dementia, this protocol aims to describe a method of imaging and analyzing retinal vasculature and neuronal structure in vivo using retinal imaging techniques. This protocol also provides examples of retinal changes in subjects with dementia, and discusses technical issues and current limitations of retinal imaging.
Protokół
All methods described here have been approved by a local clinical research ethics committee in Hong Kong.
Note: For simplicity, the equipment listed in the Table of Materials is used to illustrate the procedures of retinal imaging and subsequent analysis. Measurement of retinal vascular parameters is illustrated using the Singapore I Vessel Assessment program (SIVA) 17 (Version 4.0, National University of Singapore, Singapore). However, it should be noted that a different set of equipment can be adopted as the underlying principles remain similar.
1. Prepare the Subjects for Retinal Imaging
- Dilate the subjects’ pupils using a mydriatic agent. Wait for at least 15 min to establish sufficient pupil dilation.
2. Measure Retinal Vascular Parameters from Fundus Photographs Using a Computer-assisted Analysis program
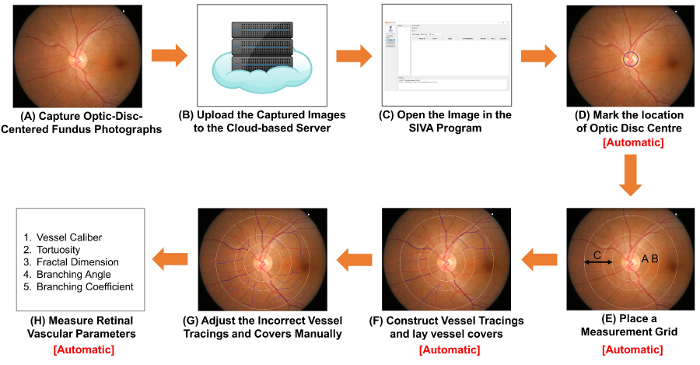
Figure 1: Schematic diagram showing the procedures of measuring retinal vascular parameters. (A) Obtain optic-disc-centered fundus photographs using a fundus camera. Figure 1A and Figure 2A are two fundus photographs with optimal quality. (B) Upload the fundus photographs to the cloud-based server and enter relevant study details, including the image conversion factor (ICF). Other computer-assisted analysis programs may use non-cloud-based methods to organize and store the images. (C) Open the fundus photograph in the computer-assisted analysis program. (D) Mark the location of optic disc center, and (E) prompt the software to automatically detect the rim of optic disc and place a measurement grid. (F) Construct vessel tracings based on the vessel paths, and lay vessel covers to estimate the diameters of the vessels. (G) Adjust the incorrect vessel tracings and vessel covers manually. (H) Measure a spectrum of retinal vascular parameters, including vessel calibers, tortuosity, fractal dimension and bifurcation. Step (D) to Step (F), and Step (H) can be automatically performed by some computer-assisted analysis programs. Please click here to view a larger version of this figure.
- Capture fundus photographs using a fundus camera.
- Turn on the fundus camera and launch the image capturing program on the computer. Rest the chin of the subject properly on the chinrest with the forehead against the head strap. Move the control lever to align the light beam properly to the subject’s pupil.
- Align the illumination points until both appear smallest on both sides in the viewfinder. Move the external fixation target to guide the subject’s eyes until the optic disc is at the center of the viewfinder and the regions of interest (ROI) are well within the boundaries. Adjust the focusing knob to focus on the retina.
- Have the subject firmly look at the external fixation target and ensure the subject’s eyes are not filled with tears.
- Depress the shutter-release button to capture an image (Figure 1A).
- Check the quality of the fundus photograph captured, using Figure 2A as a standard. Discard the image and repeat the image acquisition process (i.e., Step 2.1.1 to 2.1.4) if the pupil is poorly dilated (Figure 2B), the optic disc is not at the center of the image (Figure 2C), or the image is out of focus (Figure 2D).
- Save the image in TIFF format with gradable resolution (i.e., approximately 3,000 pixels x 2,000 pixels, at more than 150 dpi).
Note: The protocol can be paused here. - Repeat Steps 2.1.1 to 2.1.6 to acquire fundus photographs for other subjects.
- Select a 10% sample of images randomly and measure the height of optic discs in these images (Figure 3). Calculate the image conversion factor (ICF) using the formula:
ICF = 1,800 µm/(Average pixel height of optic discs of the images sampled). - Upload the captured fundus photographs to the cloud-based server and enter relevant study details, including the image conversion factor (ICF) (Figure 1B).
Note: The protocol can be paused here. Other computer-assisted analysis programs may use other non-cloud-based methods to organize the images and record the ICF.
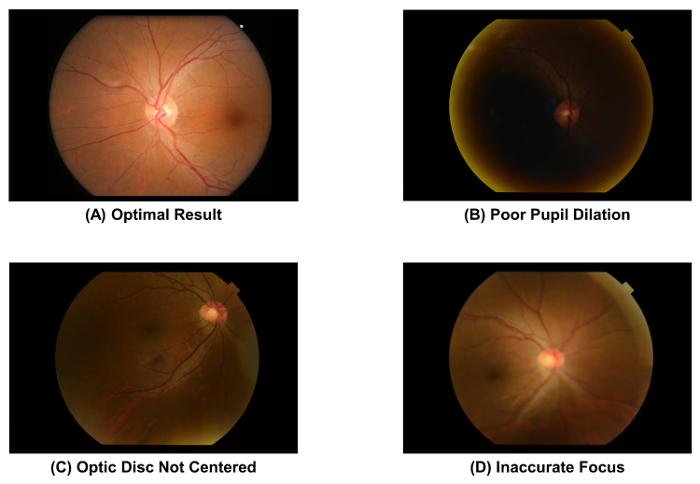
Figure 2: Fundus photographs with optimal and suboptimal quality. The image quality of a fundus photograph must be checked immediately after image acquisition, as the image quality directly affects the subsequent measurement of retinal vascular parameters. The image should be discarded if one of these artefacts is observed. These images were captured using a 50° fundus camera. Please click here to view a larger version of this figure.
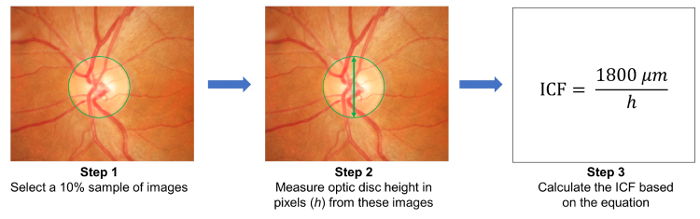
Figure 3: Calculation of the image conversion factor (ICF). To calculate the ICF, randomly select a 10% sample of images from the study (Step 1). Then, measure the height of optic discs (in pixels) from the images sampled (Step 2). Calculate the ICF using the formula: ICF= 1800 µm / (Average pixel height of optic discs of the sampled images), where 1800 µm is approximately the height of a normal optic disc (Step 3). As magnification effect and image resolution differ from camera to camera, it is necessary to calculate an accurate ICF for each camera used. Please click here to view a larger version of this figure.
- Open the fundus photograph in a computer-assisted analysis program. Construct vessel tracings and lay vessel covers for the retinal vasculature.
Note: In this section, the SIVA program is used to illustrate the procedures. However, the SIVA program can be substituted by other available computer-assisted analysis programs. In addition, Steps 2.2.2 to 2.2.3 are automatically performed by some computer-assisted analysis programs when a fundus photograph is opened (i.e. Step 2.2.1).- Open the fundus photograph with the computer-assisted analysis program (Figure 1C).
- Mark the location of the optic disc centre (Figure 1D).
- Click the “OD Center” button on the left function panel; the mouse cursor will be replaced by a green circle.
- Move the green circle to the center of the optic disc (OD), and left-click to fix the circle.
- Prompt the software to automatically place a measurement grid, construct vessel tracings and lay vessel covers (Figure 1E and 1F).
Note: Vessel covers are measurement lines that estimate the approximate width of the internal lumens of the vessels.- Click the “Find OD” button to prompt the software to detect the OD rim and place four concentric circles as a measurement grid, based on the position of the OD center.
- Click the “Process” button to initiate the automatic vessel tracing process.
- Adjust incorrect vessel tracings manually. Begin the inspection from the 12 o’clock position in a clockwise manner to ensure that all vessel tracings are verified.
- Check that the optic disc is accurately detected and the measurement grid is correctly placed. Adjust the measurement grid manually following steps 2.2.2 to 2.2.3, if the innermost circle does not accurately outline the optic disc rim (Figure 4A).
- Left click to select vessel tracing(s) labelled with incorrect vessel type (arterioles versus venules) and click the “Vessel (T)ype” button to change the vessel type.
Note: Arterioles are labelled in red and venules are labelled in blue. Arterioles can be distinguished from venules based on their physiological differences. For example, venules are generally darker in color and wider than arterioles. Vessels with same vessel type usually do not cross each other. - Extend incomplete vessel tracings following steps 2.3.3.1 to 2.3.3.2 (Figure 4B).
- Use the cursor to click at the distal end of the incomplete vessel tracing. Left click at points along the vessel path to extend the vessel tracing.
- Stop the tracing process when the distal end of the vessel is reached. Stop the tracing at the outermost white circle if the distal part of the vessel falls outside the measurement grid (see Figure 4B).
- Adjust vessel tracings if the vessel paths are not traced correctly at the crossover site (Figure 4C).
- Click the “Select” button and then click at the incorrect point of the vessel tracing. Click the “Brea(k) Seg” button to disconnect the vessel tracing at the point selected. Select the disconnected segment and click the “(Del) Seg” button to delete it.
- Re-construct a new vessel tracing using steps 2.3.3.1 and 2.3.3.2.
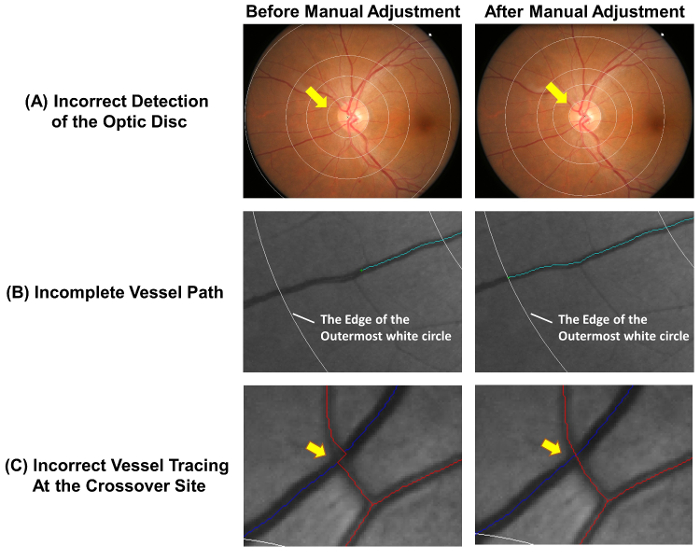
Figure 4: Common errors of the auto-tracing. The automatic vessel tracing is not completely accurate and manual adjustments are required to ensure the accuracy of measurement. This figure shows common errors of the auto-tracing and demonstrates optimal results after manual adjustments. (A) The optic disc center is incorrectly marked and this lead to deviation of the measurement grid, which may affect the subsequent measurements. Ideally, the innermost circle of the measurement grid should outline the optic disc rim. (B) Incomplete vessel tracing could lead to the incorrect measurement of fractal dimension, tortuosity, etc. The vessel path should be traced until the end of the vessel. If the distal part of the vessel falls outside the measurement grid, the tracing can be stopped at the outermost white circle. (C) Vessel tracings at the crossover sites are subject to a higher tendency of error and thus require special attention. Please click here to view a larger version of this figure.
- Lay vessel covers on all vessel segments and deactivate the incorrect covers manually.
- Click the “Find Covers” button to lay vessel covers on all vessel segments automatically.
- Check if all vessel covers are correctly placed. Left-click and drag the cursor to deactivate vessel covers if the covers are not laid perpendicular to the vessel walls (Figure 5A), the vessel path is obscured by another vessel (Figure 5B), or the covers overestimate or underestimate the width of the vessel lumen (Figure 5C).
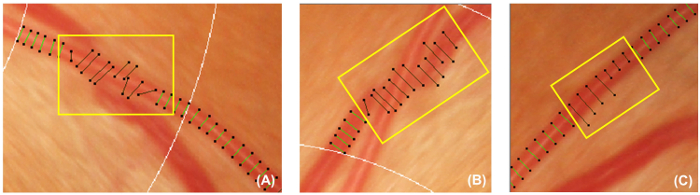
Figure 5: Incorrect vessel covers. This figure shows examples of incorrect vessel covers that should be deactivated and excluded from the subsequent measurement. Vessel covers should be deactivated if they are not perpendicular to the vessels (A). In addition, Vessel covers should also be deactivated if the vessel being traced is obscured under another vessel (B), or the vessel covers cannot represent the approximate width of the vessel (C). Please click here to view a larger version of this figure.
- Measure retinal vascular parameters from the vessel tracings and the vessel covers
Note: Step 2.5 is performed automatically by a computer-assisted analysis program.- Label the area 0.5-1.0 disc diameters away from the optic disc margin as zone B, and the area 0.5-2.0 disc diameters away from the optic disc margin as zone C18 (Figure 6A), according to the modified protocol of Atherosclerosis Risk in Communities (ARIC) study19.
- Measure retinal vascular caliber from both zone B and zone C, using a widely-adopted method that is modified from the ARIC study19,20,21,22,23,24,25,26 (Figure 6B).
- Measure the lengths of vessel covers in the six largest arterioles and the six largest venules to estimate retinal vessel calibers.
- Summarize the retinal arteriolar and venular calibers as central retinal artery equivalent (CRAE) and central retinal vein equivalent (CRVE) respectively17, using the revised Knudtson–Parr-Hubbard formula18,19.
- Identify all vessels in zone C with a width >40 µm. Calculate the retinal arteriolar and venular tortuosity from the integral of the total squared curvature along the vessel tracings and normalize the value with the total arc length, bowing, and points of inflection27,28.
- Compute the total, arteriolar, and venular fractal dimensions from zone C, using the established “box-counting method”29,30,31.
- Divide the image into a series of equally sized squares.
- Count the number of boxes containing a section of the vessel tracings.
- Repeat the process using a series of equally sized squares with different sizes.
- Plot the logarithm of the number of boxes containing the vessel tracings against the logarithm of the size of the boxes, and calculate the slope of the resulting line; this is the fractal dimension.
- Identify vessels with first bifurcation in zone C and calculate the angles (θ) subtended between the first two daughter vessels32 (Figure 6C). Compute the mean value to obtain the average branching angle.
- Calculate the branching coefficient from zone C using the formula:
(d12 + d22)/d02, where d0 is the mean trunk caliber, and d1 and d2 are the mean branch calibers (Figure 6C).
- Close the grading window. Click “send” in the pop-up dialog to upload the graded image to the cloud-based server and record the automatically measured retinal vascular parameters.
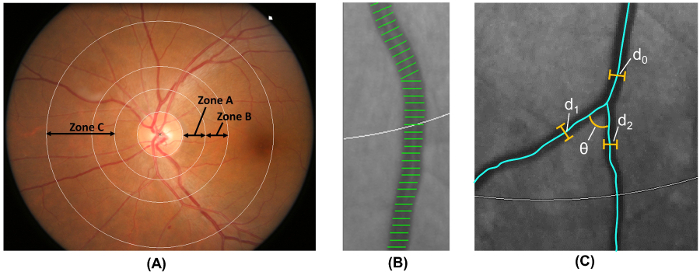
Figure 6: Quantification of retinal vasculature. (A) Zone B (defined as 0.5-1.0 disc diameters away from the disc margin) is used to measure vessel calibers of zone B according to the Atherosclerosis Risk in Communities Study. Zone C (defined as 0.5-2.0 disc diameters away from the disc margin) is used to measure vessel calibers of zone C and a spectrum of retinal vascular network parameters (such as tortuosity, fractal dimension, and bifurcation). (B) Vessel covers are measurement lines used to estimate the retinal vessel calibers (or diameters). Incorrect vessel covers should be manually excluded from the measurement. (C) For all vessels that have their first bifurcation within zone C, the program automatically measures the branching angles (θ) of the first bifurcation. In addition, the branching coefficient is also calculated using the formula: Branching coefficient = (d12 + d22)/d02, where d0 is the trunk caliber, and d1 and d2 are the branch calibers. Please click here to view a larger version of this figure.
3. Assess the Thickness of GC-IPL and RNFL
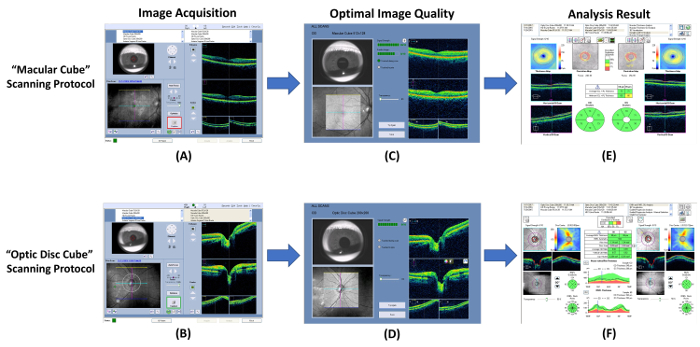
Figure 7: Schematic diagram showing the procedures of measuring RNFL and GC-IPL thickness. Optical coherence tomography (OCT) can be used to measure thicknesses of the ganglion cell-inner plexiform layer (GC-IPL) and the retinal nerve fiber layer (RNFL). (A, B) Measure the GC-IPL and RNFL thicknesses using the built-in “macular cube” and “optic disc cube” scanning protocols respectively. (C, D) Check the image quality immediately after image acquisition. Discard the image and repeat the scan if the signal strength is smaller than 6, or motion artefacts are detected. (E, F) Then, prompt the built-in analysis program to automatically analyze the scan result and generate a report for interpretation. Please click here to view a larger version of this figure.
- Perform image acquisition using optical coherence tomography (OCT).
- Open the OCT program and select the “Macular Cube” scanning protocol to start a new macular scan (Figure 7A).
- Locate the pupil in the iris viewport by adjusting the chinrest. Lower the illumination if the pupil size is too small.
- Click the “Auto Focus” button and then the “Optimize” button to improve the image quality.
- Instruct the subject to blink a few times immediately before starting the scan.
- Click the “Capture” button to start the scan when the border surrounding the button becomes green. Instruct the subject to focus on the visual fixation target during image acquisition to avoid motion artifacts.
- Review the scan quality using Figure 7C as a standard. Discard the scan result and repeat the scan if the signal strength is smaller than 6 (Figure 8A), or motion artefacts are detected (indicated by discontinuity of blood vessels) (Figure 8B).
- Save the scan result.
- Repeat Steps 3.1.1 to 3.1.7 for another eye.
- Perform an optic nerve head scan with the “Optic Disc Cube” scanning protocol following steps 3.1.2 to 3.1.9 (Figures 7B and 7D).

Figure 8: Sub-optimal results of optical coherence tomography. Common sub-optimal results of the optical coherence tomography (OCT) include (A) poor signal strength (strength value <6), and (B) motion artefacts. The scan quality should be reviewed immediately after image acquisition, and the scan should be repeated if these artefacts are encountered. Please click here to view a larger version of this figure.
- Generate an analysis printout of the macular GC-IPL thickness.
- Select the “Macular Cube” scan records of both eyes in the analysis interface.
- Click the “Ganglion Cell OU Analysis” to initiate the automatic analysis algorithm to assess the GC-IPL thickness of the scan (Figure 7E).
Note: Step 3.2.2 is automatically completed by the analysis algorithm.- Generate a 14.13 mm2 fovea-centered elliptical annulus that has horizontal inner and outer radiuses of 0.6 mm and 2.4 mm, respectively, and vertical inner and outer radiuses of 0.5 mm and 2.0 mm, respectively.
Note: The size and shape of the elliptical annulus conform closely to the macular anatomy and thus correspond to the area where the RGCs are thickest in normal eyes33,34. The area within the inner ring of the annulus is not measured, as the GC-IPL in this area is very thin. - Segment the outer boundary of the RNFL and the outer boundary of the inner plexiform layer (IPL) to locate the GC-IPL (Figure 9).
- Measure the average, minimum, and six sectorial (superotemporal, superior, superonasal, inferonasal, inferior, inferotemporal) thicknesses of macular GC-IPL within the fovea-centered elliptical annulus.
- Compare the measured GC-IPL thicknesses to the device’s internal normative age-matched database and generate a deviation map and a significance map
- Report the measurement results on an analysis printout.
- Generate a 14.13 mm2 fovea-centered elliptical annulus that has horizontal inner and outer radiuses of 0.6 mm and 2.4 mm, respectively, and vertical inner and outer radiuses of 0.5 mm and 2.0 mm, respectively.
- Save the analysis printout in the .pdf format.
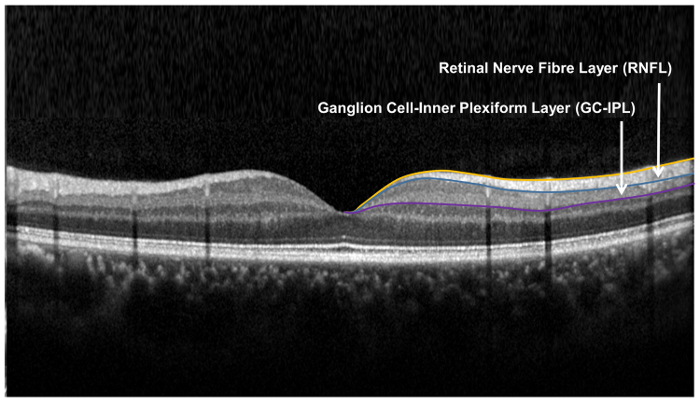
Figure 9: Retinal layers used for the assessment of the retinal neuronal structure. The retinal nerve fibre layer (RNFL) is measured using the optic nerve head (ONH) algorithm, while the ganglion cell-inner plexiform layer is measured using the ganglion cell analysis (GCA) algorithm. The ONH algorithm segments the inner and outer boundary of the RNFL to measure the thickness of RNFL. The GCA algorithm detects the outer boundary of the retinal nerve fiber layer (RNFL) and the inner plexiform layer (IPL) to yield the combined thickness of the ganglion-cell layer (GCL) and the IPL. The thicknesses of GCL and the IPL are measured together, as the boundary between GCL and IPL is anatomically indistinct. However, the combined thickness of GCL and IPL (i.e. GC-IPL) is still indicative of the health of RGCs. Please click here to view a larger version of this figure.
- Generate the analysis printout of the RNFL thickness (Figure 7F).
- Select the “Optic Disc Cube” scan records of both eyes in the analysis interface.
- Click the “ONH and RNFL OU Analysis” to initiate the automatic analysis algorithm to assess the RNFL thickness of the scan.
Note: Steps 3.3.2.1 to 3.3.2.6 can be automatically completed by the analysis algorithm.- Measure the RNFL thickness at each scan point and generate an RNFL thickness map.
- Identify the optic disc by detecting a dark spot near the center of the scan that has a size and shape consistent with the range of an optic disc.
- Position a measurement grid of 3.46 mm in diameter around the optic disc on the RNFL thickness map.
- Measure and calculate the global, four-quadrants (temporal, superior, nasal and inferior), and twelve-clock-hour parapapillary RNFL thicknesses of the measurement grid.
- Compare the measured RNFL thicknesses to the device’s internal normative age-matched database and generate a deviation map and a significance map.
- Report the measurement results on an analysis printout.
- Save the analysis printout in the .pdf format.
Wyniki
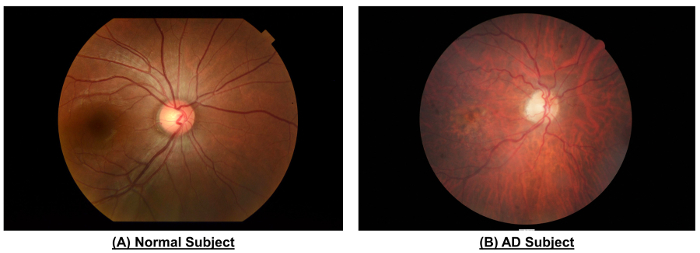
Figure 10: An example to show the differences in retinal vasculature between a normal subject and an AD subject. When compared to the normal subject, fundus photograph of the AD subject showed narrower vessel calibers (CRAE of Zone B, 116.4 µm vs. 156.4 µm; CRVE of Zone B, 186.9 µm vs. 207.5 µm; CRAE of Zone C, 138....
Dyskusje
This protocol describes the procedures of quantifying neuronal and vascular changes in the retina in vivo. As the retina shares similar embryological origins, anatomical features and physiological properties with the brain, these retinal changes may reflect similar changes of vasculature and neuronal structure in the brain.
As shown in Figure 10 and Table 1, the AD subject showed decreased vessel calibers when compared to the healt...
Ujawnienia
Regarding potential financial ties, the author Tien Y. Wong is a co-inventor of the Singapore I Vessel Assessment (SIVA) program used in this article.
Podziękowania
We would like to express our appreciation to the School of Computing, National University of Singapore for technical support and the Health and Medical Research Fund (04153506), Hong Kong for funding support.
Materiały
| Name | Company | Catalog Number | Comments |
| Non-mydriatic Retinal Camera | Topcon, Inc, Tokyo, Japan | TRC 50DX | N/A |
| Singapore I Vessel Assessment Program | National University of Singapore | Version 4.0 | N/A |
| CIRRUS HD-OCT | Carl Zeiss Meditec, Inc, Dublin, CA | Model 4000 | N/A |
| Mydriatic Agents | N/A | N/A | Prepared from 1% tropicamide and 2.5% phenylephrine hydrochloride |
Odniesienia
- Alzheimer's Disease International. The prevalence of dementia worldwide. Alzheimer's Dis. Int. (December), 1-2 (2008).
- Wimo, A., Winblad, B., &Jönsson, L. The worldwide societal costs of dementia: Estimates for 2009. Alzheimer's Dement. 6 (2), 98-103 (2010).
- Comas-Herrera, A., Northey, S., Wittenberg, R., Knapp, M., Bhattacharyya, S., Burns, A. Future costs of dementia-related long-term care: exploring future scenarios. Int. Psychogeriatr. 23 (1), 20-30 (2011).
- Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimer's Dement. 10 (2), e47-e92 (2014).
- Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., Ferri, C. P. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers. Dement. 9 (1), 63-75 (2013).
- Alzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimer's Dement. 12 (4), 459-509 (2016).
- Asih, P. R., Chatterjee, P., Verdile, G., Gupta, V. B., Trengove, R. D., Martins, R. N. Clearing the amyloid in Alzheimer's: progress towards earlier diagnosis and effective treatments - an update for clinicians. Neurodegener. Dis. Manag. 4 (5), 363-378 (2014).
- Cheung, C. Y., Ikram, M. K., Chen, C., Wong, T. Y. Imaging retina to study dementia and stroke. Prog. Retin. Eye Res. , (2017).
- Patton, N., Aslam, T., Macgillivray, T., Pattie, A., Deary, I. J., Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 206 (4), 319-348 (2005).
- London, A., Benhar, I., Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 9 (1), 44-53 (2013).
- Crowe, M. J., Bresnahan, J. C., Shuman, S. L., Masters, J. N., Beattie, M. S. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 3 (1), 73-76 (1997).
- Levkovitch-Verbin, H., Quigley, H. A., Kerrigan-Baumrind, L. A., D'Anna, S. A., Kerrigan, D., Pease, M. E. Optic nerve transection in monkeys may result in secondary degeneration of retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 42 (5), 975-982 (2001).
- Levkovitch-Verbin, H., Quigley, H. A., Martin, K. R., Zack, D. J., Pease, M. E., Valenta, D. F. A model to study differences between primary and secondary degeneration of retinal ganglion cells in rats by partial optic nerve transection. Invest Ophthalmol Vis Sci. 44 (8), 3388-3393 (2003).
- Yoles, E., Schwartz, M. Degeneration of spared axons following partial white matter lesion: implications for optic nerve neuropathies. Exp Neurol. 153 (1), 1-7 (1998).
- Sadun, A. A., Borchert, M., DeVita, E., Hinton, D. R., Bassi, C. J. Assessment of Visual Impairment in Patients With Alzheimer's Disease. Am. J. Ophthalmol. 104 (2), 113-120 (1987).
- Schlotterer, G., Moscovitch, M., Crapper-Mclachlan, D. Visual processing deficits as assessed by spatial frequency contrast sensitivity and backward masking in normal ageing and alzheimer's. Brain. 107 (1), 309-324 (1984).
- Cheung, C. Y. L., et al. A new method to measure peripheral retinal vascular caliber over an extended area. Microcirculation. 17 (7), 495-503 (2010).
- Knudtson, M. D., Lee, K. E., Hubbard, L. D., Wong, T. Y., Klein, R., Klein, B. E. K. Revised formulas for summarizing retinal vessel diameters. Curr. Eye Res. 27 (3), 143-149 (2003).
- Hubbard, L. D., et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 106 (12), 2269-2280 (1999).
- Patton, N., et al. The association between retinal vascular network geometry and cognitive ability in an elderly population. Investig. Ophthalmol. Vis. Sci. 48 (5), 1995-2000 (2007).
- VanHecke, M. V., et al. Are retinal microvascular abnormalities associated with large artery endothelial dysfunction and intima-media thickness? The Hoorn Study. Clin. Sci. London Engl. 110 (5), 597-604 (2006).
- Tien, Y. W., et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: The Multi-Ethnic Study of Atherosclerosis (MESA). Investig. Ophthalmol. Vis. Sci. 47 (6), 2341-2350 (2006).
- Leung, H., et al. Relationships between age, blood pressure, and retinal vessel diameters in an older population. Investig. Ophthalmol. Vis. Sci. 44 (7), 2900-2904 (2003).
- Wong, T. Y., et al. The prevalence and risk factors of retinal microvascular abnormalities in older persons: The cardiovascular health study. Ophthalmology. 110 (4), 658-666 (2003).
- Ikram, M. K., et al. Retinal vessel diameters and risk of stroke: The Rotterdam Study. Neurology. 66 (9), 1339-1343 (2006).
- Wong, T. Y., Knudtson, M. D., Klein, R., Klein, B. E. K., Meuer, S. M., Hubbard, L. D. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: Methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 111 (6), 1183-1190 (2004).
- Sasongko, M. B., et al. Alterations in retinal microvascular geometry in young type 1 diabetes. Diabetes Care. 33 (6), 1331-1336 (2010).
- Cheung, C. Y. L., et al. Retinal vascular tortuosity, blood pressure, and cardiovascular risk factors. Ophthalmology. 118 (5), 812-818 (2011).
- Mainster, M. a The fractal properties of retinal vessels: embryological and clinical implications. Eye. 4 ( Pt 1) (1), 235-241 (1990).
- Liew, G., et al. The Retinal Vasculature as a Fractal: Methodology, Reliability, and Relationship to Blood Pressure. Ophthalmology. 115 (11), (2008).
- Stosic, T., Stosic, B. D. Multifractal analysis of human retinal vessels. IEEE Trans. Med. Imaging. 25 (8), 1101-1107 (2006).
- Zamir, M., Medeiros, J. A., Cunningham, T. K. &. a. m. p. ;. M., Zamir, J. A., Medeiros, T. K. C. Arterial bifurcations in the human retina. J. Gen. Physiol. 74 (4), 537-548 (1979).
- Mwanza, J. C., Oakley, J. D., Budenz, D. L., Chang, R. T., Knight, O. J., Feuer, W. J. Macular ganglion cell-inner plexiform layer: Automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Investig. Ophthalmol. Vis. Sci. 52 (11), 8323-8329 (2011).
- Bendschneider, D., et al. Retinal nerve fiber layer thickness in normals measured by spectral domain OCT. J. Glaucoma. 19 (7), 475-482 (2010).
- Cheung, C. Y., Ong, Y. T., Ikram, M. K., Chen, C., Wong, T. Y. Retinal Microvasculature in Alzheimer's Disease. J. Alzheimer's Dis. 42 (s4), S339-S352 (2014).
- Murray, C. D. THE PHYSIOLOGICAL PRINCIPLE OF MINIMUM WORK APPLIED TO THE ANGLE OF BRANCHING OF ARTERIES. J. Gen. Physiol. (4), 835-841 (1926).
- Ding, J., et al. Early retinal arteriolar changes and peripheral neuropathy in diabetes. Diabetes Care. 35 (5), 1098-1104 (2012).
- Yim, C., et al. Retinal Ganglion Cell Analysis Using High-Definition Optical Coherence Tomography in Patients with Mild Cognitive Impairment and Alzheimer's Disease. J. Alzheimer's Dis. Retin. Ganglion Cell Anal. MCI AD. 45 (1), 45-56 (2015).
- Stein, D. M., Wollstein, G., Ishikawa, H., Hertzmark, E., Noecker, R. J., Schuman, J. S. Effect of Corneal Drying on Optical Coherence Tomography. Ophthalmology. 113 (6), 985-991 (2006).
- Mwanza, J. C., et al. Effect of Cataract and its Removal on Signal Strength and Peripapillary Retinal Nerve Fiber Layer Optical Coherence Tomography Measurements. J. Glaucoma. 20 (1), 37-43 (2011).
- Garcia-Martin, E., et al. Influence of cataract surgery on optical coherence tomography and neurophysiology measurements in patients with retinitis pigmentosa. Am. J. Ophthalmol. 156 (2), (2013).
- Kok, P. H. B., et al. The relationship between the optical density of cataract and its influence on retinal nerve fibre layer thickness measured with spectral domain optical coherence tomography. Acta Ophthalmol. , (2012).
- Kim, N. R., et al. Influence of cataract on time domain and spectral domain optical coherence tomography retinal nerve fiber layer measurements. J. Glaucoma. 21 (2), 116-122 (2012).
- Hwang, Y. H., Kim, Y. Y. Effect of Peripapillary Vitreous Opacity on Retinal Nerve Fiber Layer Thickness Measurement Using Optical Coherence Tomography. Arch. Ophthalmol. 130 (6), 789-792 (2012).
- Schwartz, S. G., Flynn, H. W., Fisher, Y. L. "Floater scotoma" demonstrated on spectral-domain optical coherence tomography and caused by vitreous opacification. Ophthalmic Surg. Lasers Imaging Retina. 44 (4), 415-418 (2013).
- Frost, S., et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer's disease. Transl. Psychiatry. 3 (2), e233 (2013).
- Cheung, C. Y., et al. Microvascular network alterations in the retina of patients with Alzheimer's disease. Alzheimer's Dement. 10 (2), 135-142 (2014).
- DeJong, F. J., et al. Retinal vascular caliber and risk of dementia: The Rotterdam Study. Neurology. 76 (9), 816-821 (2011).
- Cheung, C. Y., et al. Quantitative and qualitative retinal microvascular characteristics and blood pressure. J. Hypertens. 29 (7), 1380-1391 (2011).
- Cheung, C. Y., et al. Retinal vascular fractal dimension and its relationship with cardiovascular and ocular risk factors. Am. J. Ophthalmol. 154 (4), 663-674 (2012).
- Cheung, C. Y. L., et al. Retinal vascular tortuosity, blood pressure, and cardiovascular risk factors. Ophthalmology. 118 (5), 812-818 (2011).
- Grinton, M. E., et al. The association between retinal vessel morphology and retinal nerve fiber layer thickness in an elderly population. Ophthalmic Surg. Lasers Imaging. 43 (6 Suppl), S61-S66 (2012).
- Hughes, A. D., et al. Quantification of topological changes in retinal vascular architecture in essential and malignant hypertension. J. Hypertens. 24 (5), 889-894 (2006).
- Hughes, A. D., et al. Determinants of retinal microvascular architecture in normal subjects. Microcirculation. 16 (2), 159-166 (2009).
- Lau, Q. P., Lee, M. L., Hsu, W., Wong, T. Y. The Singapore Eye Vessel Assessment System. Image Anal. Model. Ophthalmol. , 143-160 (2014).
- Thomas, G. N., et al. Measurement of Macular Fractal Dimension Using a Computer-Assisted Program. Investig. Opthalmology Vis. Sci. 55 (4), 2237 (2014).
- Murray, C. D. The physiological principle of minimal work. I. The vascular system and the cost of blood volume. Proc. Natl. Acad. Sci. 12, 207-214 (1926).
- Cheung, C., Chen, C., Wong, T. Ocular Fundus Photography as a Tool to Study Stroke and Dementia. Semin. Neurol. 35 (5), 481-490 (2015).
- Williams, M. A., et al. Retinal microvascular network attenuation in Alzheimer's disease. Alzheimer's Dement. Diagnosis, Assess. Dis. Monit. 1 (2), 229-235 (2015).
- Cheung, C. Y., et al. Retinal Vascular Fractal Dimension Is Associated with Cognitive Dysfunction. J. Stroke Cerebrovasc. Dis. 23 (1), 43-50 (2014).
- Hammes, H. P., et al. Diabetic retinopathy: targeting vasoregression. Diabetes. 60 (1), 9-16 (2011).
- Cheung, C. Y., et al. Microvascular network alterations in the retina of patients with Alzheimer's disease. Alzheimer's Dement. 10 (2), 135-142 (2014).
- Frame, M. D., Sarelius, I. H. Arteriolar bifurcation angles vary with position and when flow is changed. Microvasc Res. 46 (2), 190-205 (1993).
- Djonov, V., Baum, O., Burri, P. H. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 314 (1), 107-117 (2003).
- Griffith, T. M., Edwards, D. H. Basal EDRF activity helps to keep the geometrical configuration of arterial bifurcations close to the Murray optimum. J. Theor. Biol. 146 (4), 545-573 (1990).
- Griffith, T. M., Edwards, D. H., Randall, M. D. Blood flow and optimal vascular topography: role of the endothelium. Basic Res. Cardiol. 86 Suppl 2, 89-96 (1991).
- Chapman, N., Haimes, G., Stanton, A. V., Thom, S. A. M., Hughes, A. D. Acute effects of oxygen and carbon dioxide on retinal vascular network geometry in hypertensive and normotensive subjects. Clin. Sci. 99 (6), 483-488 (2000).
- Heringa, S. M., Bouvy, W. H., van denBerg, E., Moll, A. C., Jaap Kappelle, L., Jan Biessels, G. Associations between retinal microvascular changes and dementia, cognitive functioning, and brain imaging abnormalities: a systematic review. J. Cereb. blood flow Metab. 33 (7), 983-995 (2013).
- Ding, J., et al. Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: The Edinburgh type 2 diabetes study. Diabetes. 59 (11), 2883-2889 (2010).
- Parisi, V., Restuccia, R., Fattapposta, F., Mina, C., Bucci, M. G., Pierelli, F. Morphological and functional retinal impairment in Alzheimer's disease patients. Clin. Neurophysiol. 112 (10), 1860-1867 (2001).
- Paquet, C., Boissonnot, M., Roger, F., Dighiero, P., Gil, R., Hugon, J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Neurosci. Lett. 420 (2), 97-99 (2007).
- Moschos, M. M., et al. Structural and functional impairment of the retina and optic nerve in Alzheimer's disease. Curr. Alzheimer Res. 9 (7), 782-788 (2012).
- Lu, Y., et al. Retinal nerve fiber layer structure abnormalities in early Alzheimer's disease: Evidence in optical coherence tomography. Neurosci. Lett. 480 (1), 69-72 (2010).
- Kesler, A., Vakhapova, V., Korczyn, A. D., Naftaliev, E., Neudorfer, M. Retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Clin. Neurol. Neurosurg. 113 (7), 523-526 (2011).
- Ascaso, F. J., et al. Retinal alterations in mild cognitive impairment and Alzheimer's disease: An optical coherence tomography study. J. Neurol. 261 (8), 1522-1530 (2014).
- Berisha, F., Feke, G. T., Trempe, C. L., McMeel, J. W., Schepens, C. L. Retinal abnormalities in early Alzheimer's disease. Investig. Ophthalmol. Vis. Sci. 48 (5), 2285-2289 (2007).
- Iseri, P. K., Altinaş, O., Tokay, T., Yüksel, N. Relationship between Cognitive Impairment and Retinal Morphological and Visual Functional Abnormalities in Alzheimer Disease. J. Neuro-Ophthalmology. 26 (1), 18-24 (2006).
- Garcia-Martin, E. S., et al. Macular thickness as a potential biomarker of mild Alzheimer's disease. Ophthalmology. 121 (5), 1149-1151 (2014).
- Ko, F., et al. Retinal Nerve Fiber Layer Thinning Associated With Poor Cognitive Function Among A Large Cohort, The Uk Biobank. Alzheimer's Dement. 12 (7), P317-P318 (2016).
- Moreno-Ramos, T., Benito-Leon, J., Villarejo, A., Bermejo-Pareja, F. Retinal nerve fiber layer thinning in dementia associated with Parkinson's disease, dementia with Lewy bodies, and Alzheimer's disease. J. Alzheimers. Dis. 34 (3), 659-664 (2013).
- Moschos, M. M., et al. Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur. J. Ophthalmol. 21 (1), 24-29 (2011).
- Garcia-Martin, E., et al. Ability and reproducibility of Fourier-domain optical coherence tomography to detect retinal nerve fiber layer atrophy in Parkinson's disease. Ophthalmology. 119 (10), 2161-2167 (2012).
- Yip, W., et al. Comparison of Common Retinal Vessel Caliber Measurement Software and a Conversion Algorithm. Transl. Vis. Sci. Technol. 5 (5), 11 (2016).
- Gorelick, P. B., et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 42 (9), 2672-2713 (2011).
- Brown, W. R., Thore, C. R. Review: Cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol. Appl. Neurobiol. 37 (1), 56-74 (2011).
- DeSilva, T. M., Faraci, F. M. Microvascular Dysfunction and Cognitive Impairment. Cell. Mol. Neurobiol. 36 (2), 241-258 (2016).
- Kalaria, R. N., Akinyemi, R., Ihara, M. Does vascular pathology contribute to Alzheimer changes?. J. Neurol. Sci. 322 (1-2), 141-147 (2012).
- Kling, M. A., Trojanowski, J. Q., Wolk, D. A., Lee, V. M. Y., Arnold, S. E. Vascular disease and dementias: paradigm shifts to drive research in new directions. Alzheimers. Dement. 9 (1), 76-92 (2013).
- O'Brien, J. T., et al. Vascular cognitive impairment. Lancet Neurol. 2 (2), 89-98 (2003).
- Chen, C., et al. Alzheimer's disease with cerebrovascular disease: current status in the Asia-Pacific region. J. Intern. Med. 280 (4), 359-374 (2016).
- Pérez, M. A., Bruce, B. B., Newman, N. J., Biousse, V. The use of retinal photography in nonophthalmic settings and its potential for neurology. Neurologist. 18 (6), 350-355 (2012).
- Boppart, S. A. Optical coherence tomography: Technology and applications for neuroimaging. Psychophysiology. 40 (4), 529-541 (2003).
- Hee, M. R., et al. Optical coherence tomography of the human retina. Arch. Ophthalmol. 113 (3), 325-332 (1995).
- Huang, D., et al. Optical coherence tomography. Science (80-.). 254 (5035), 1178-1181 (1991).
- vanVelthoven, M. E. J., Verbraak, F. D., Yannuzzi, L., Rosen, R. B., Podoleanu, A. G. H., deSmet, M. D. Imaging the retina by en face optical coherence tomography. Retina. 26 (2), 129-136 (2006).
- Costa, R. A., et al. Retinal assessment using optical coherence tomography. Prog. Retin. Eye Res. 25 (3), 325-353 (2006).
- DeBuc, D. C., Somfai, G. M., Ranganathan, S., Tátrai, E., Ferencz, M., Puliafito, C. A. Reliability and reproducibility of macular segmentation using a custom-built optical coherence tomography retinal image analysis software. J. Biomed. Opt. 14 (6), 64023 (2009).
- Budenz, D. L., et al. Determinants of Normal Retinal Nerve Fiber Layer Thickness Measured by Stratus OCT. Ophthalmology. 114 (6), 1046-1052 (2007).
- Leung, C. K. S., et al. Retinal Nerve Fiber Layer Imaging with Spectral-Domain Optical Coherence Tomography: A Prospective Analysis of Age-Related Loss. Ophthalmology. 119 (4), 731-737 (2012).
- Cettomai, D., et al. Reproducibility of optical coherence tomography in multiple sclerosis. Arch. Neurol. 65 (9), 1218-1222 (2008).
- Garcia-Martin, E., Pinilla, I., Idoipe, M., Fuertes, I., Pueyo, V. Intra and interoperator reproducibility of retinal nerve fibre and macular thickness measurements using Cirrus Fourier-domain OCT. Acta Ophthalmol. 89 (1), (2011).
- Garcia-Martin, E., Pueyo, V., Pinilla, I., Ara, J. R., Martin, J., Fernandez, J. Fourier-domain OCT in multiple sclerosis patients: reproducibility and ability to detect retinal nerve fiber layer atrophy. Invest. Ophthalmol. Vis. Sci. 52 (7), 4124-4131 (2011).
- Menke, M. N., Knecht, P., Sturm, V., Dabov, S., Funk, J. Reproducibility of nerve fiber layer thickness measurements using 3D fourier-domain OCT. Invest. Ophthalmol. Vis. Sci. 49 (12), 5386-5391 (2008).
- Mwanza, J. C., et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Investig. Ophthalmol. Vis. Sci. 51 (11), 5724-5730 (2010).
- Syc, S. B., et al. Reproducibility of high-resolution optical coherence tomography in multiple sclerosis. Mult Scler. 16 (7), 829-839 (2010).
- Ikram, M. K., Cheung, C. Y., Wong, T. Y., Chen, C. P. L. H. Retinal pathology as biomarker for cognitive impairment and Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 83 (9), 917-922 (2012).
- MacGillivray, T. J., Trucco, E., Cameron, J. R., Dhillon, B., Houston, J. G., vanBeek, E. J. R. Retinal imaging as a source of biomarkers for diagnosis, characterization and prognosis of chronic illness or long-term conditions. Br. J. Radiol. 87 (1040), 20130832 (2014).
- Patton, N., et al. Retinal image analysis: Concepts, applications and potential. Prog. Retin. Eye Res. 25 (1), 99-127 (2006).
- McGrory, S., et al. The application of retinal fundus camera imaging in dementia: A systematic review. Alzheimer's Dement. Diagnosis, Assess. Dis. Monit. 6, 91-107 (2017).
- Wong, T. Y., Knudtson, M. D., Klein, R., Klein, B. E. K., Meuer, S. M., Hubbard, L. D. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 111 (6), 1183-1190 (2004).
- Hardin, J. S., Taibbi, G., Nelson, S. C., Chao, D., Vizzeri, G. Factors Affecting Cirrus-HD OCT Optic Disc Scan Quality: A Review with Case Examples. J. Ophthalmol. 2015, 1-16 (2015).
- Kim, N. R., et al. Influence of Cataract on Time Domain and Spectral Domain Optical Coherence Tomography Retinal Nerve Fiber Layer Measurements. J. Glaucoma. 1, (2010).
- Li, H., et al. Lens opacity and refractive influences on the measurement of retinal vascular fractal dimension. Acta Ophthalmol. 88 (6), e234-e240 (2010).
- Maberley, D., Morris, A., Hay, D., Chang, A., Hall, L., Mandava, N. A comparison of digital retinal image quality among photographers with different levels of training using a non-mydriatic fundus camera. Ophthalmic Epidemiol. 11 (3), 191-197 (2004).
- Rochtchina, E., Wang, J. J., Taylor, B., Wong, T. Y., Mitchell, P. Ethnic variability in retinal vessel caliber: A potential source of measurement error from ocular pigmentation?-The Sydney childhood eye study. Investig. Ophthalmol. Vis. Sci. 49 (4), 1362-1366 (2008).
- Wainwright, A., et al. Effect of image quality, color, and format on the measurement of retinal vascular fractal dimension. Investig. Ophthalmol. Vis. Sci. 51 (11), 5525-5529 (2010).
- Nguyen, T. T., Wong, T. Y. Retinal vascular manifestations of metabolic disorders. Trends Endocrinol. Metab. 17 (7), 262-268 (2006).
- Ding, J., et al. Retinal vascular caliber and the development of hypertension: a meta-analysis of individual participant data. J. Hypertens. 32 (2), 207-215 (2014).
- Nguyen, T. T., Wong, T. Y. Retinal vascular changes and diabetic retinopathy. Curr. Diab. Rep. 9 (4), 277-283 (2009).
- Leung, C. K. S., Ye, C., Weinreb, R. N., Yu, M., Lai, G., Lam, D. S. Impact of Age-related Change of Retinal Nerve Fiber Layer and Macular Thicknesses on Evaluation of Glaucoma Progression. Ophthalmology. 120 (12), 2485-2492 (2013).
- Sherry, L. M., et al. Reliability of computer-assisted retinal vessel measurement in a population. Clin. Experiment. Ophthalmol. 30 (3), 179-182 (2002).
- Wardlaw, J. M., et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12 (8), 822-838 (2013).
- Patton, N., Aslam, T., MacGillivray, T., Pattie, A., Deary, I. J., Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 206 (4), 319-348 (2005).
- Ferri, C. P., et al. Global prevalence of dementia: A Delphi consensus study. Lancet. 366 (9503), 2112-2117 (2005).
- Sahadevan, S., et al. Ethnic differences in Singapore's dementia prevalence: The stroke, Parkinson's disease, epilepsy, and dementia in Singapore study. J. Am. Geriatr. Soc. 56 (11), 2061-2068 (2008).
- Kernt, M., et al. Assessment of diabetic retinopathy using nonmydriatic ultra-widefield scanning laser ophthalmoscopy (Optomap) compared with ETDRS 7-field stereo photography. Diabetes Care. 35 (12), 2459-2463 (2012).
- Manivannan, A., Plskova, J., Farrow, A., Mckay, S., Sharp, P. F., Forrester, J. V. Ultra-wide-field fluorescein angiography of the ocular fundus. Am. J. Ophthalmol. 140 (3), 525-527 (2005).
- Pellegrini, E., et al. Blood vessel segmentation and width estimation in ultra-wide field scanning laser ophthalmoscopy. Biomed. Opt. Express. 5 (12), 4329 (2014).
- Estrada, R., Tomasi, C., Schmidler, S. C., Farsiu, S. Tree topology estimation. IEEE Trans. Pattern Anal. Mach. Intell. 37 (8), 1688-1701 (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone