Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Laboratory Rearing of Stable Flies and Other Muscoid Diptera
W tym Artykule
Podsumowanie
A procedure for rearing stable flies (Stomoxys calcitrans) is presented. The procedure uses locally available materials for diet components, equipment and supplies.
Streszczenie
Stable flies, Stomoxys calcitrans, are serious pests of livestock, humans, companion animals and wildlife worldwide. During the last 20+ years, changes in agronomic practices resulted in serious outbreaks of stable flies in several countries. These outbreaks disrupted livestock production and human recreation resulting in public demands for increasing research and management efforts for this pest. A simple and inexpensive procedure for rearing stable flies for laboratory studies is presented. The procedure uses locally available diet components, equipment and supplies. The procedure can be adapted for rearing other muscoid flies including face fly (Musca autumnalis), horn fly (Haematobia irritans), and house fly (Musca domestica). The procedure produces stable fly puparia averaging 12.5 mg and ~35% egg to adult survival. Approximately 3000 flies are produced in each pan.
Wprowadzenie
Stable flies, Stomoxys calcitrans (L.), are hematophagous flies whose painful bites disrupt the grazing behavior of livestock, cause pain and suffering to companion animals and disrupt human recreational activities worldwide. Immature stable flies develop in fermenting vegetative matter, often contaminated with animal waste. Changing agronomic practices and crops have produced serious outbreaks of stable flies in crop residues, vegetables in Australia1, sugar cane in Brazil2, and pineapple in Costa Rica3. Although just 14 stable flies per animal are considered to be the economic threshold4, observations of more than 2,000 flies per animal have been made during recent outbreaks5. Such infestation levels reduce host productivity to near zero and can cause mortality6. As a result of agronomically associated outbreaks, stable flies are receiving renewed interest and demand for laboratory colonies has increased dramatically.
As for all holometamorphic insects, stable flies obtain all of the nutrients required for growth during the immature or larval stage. Therefore, an important component of a rearing system is the larval diet or substrate. Stable fly larvae have been observed developing in a broad range of substrates in the field7 and they are dependent upon the microbial community of the substrate8,9. Natural larval substrates are primarily composed of decomposing or fermenting vegetative materials often contaminated with nitrogenous wastes.
For laboratory rearing, stable fly larval substrates are usually composed of a vegetative material and an added nitrogen source. Numerous materials have been used for stable fly larval diets. The first larval diets mimicked natural substrates and included fermenting oat straw and horse or cow manure10,11. Carbohydrate sources include wheat bran12,13,14, alfalfa meal12,13,14 and a commercial formulation developed by Chemical Specialties Manufacturers Association (CSMA, 33% wheat bran, 27% alfalfa meal, 40% brewer's yeast granules)13,14,15,16. Nitrogen sources include yeast suspension12, fish meal and ammonium bicarbonate17. Inert bulking materials are often included in diets including oat hulls12, bagasse13, vermiculite16, wood chips13,18 and pelleted peanut hulls14.
A primary objective of laboratory rearing is to produce a product that is as physiologically similar to "wild type" as possible in order that laboratory experiments will produce results reflecting those of field populations. This requires that in-breeding and selection be minimized to maintain genetic diversity and nutritional resources be comparable to those in the field. Secondary objectives are to minimize labor and expenses. A major component of minimizing expenses is the use of locally available diet components. The stable fly rearing system presented was developed to meet these objectives.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Egg Collection (Figure 1)
- Prepare egging cup, place one end of cloth in ~500 mL beaker filled with warm (~40 °C) water. Overlap the sides of the cup and fix with a rubber band. Fold the loose end of the cloth back over the top of the cup.
- Place egging cup in cage of 8–10 day old flies for ~2 h. Gravid female stable flies will oviposit on the cloth.
- Remove egging cup from cage and rinse eggs off of the egging cloth into a small pan with squeeze wash bottle.
2. Prepare Larval Rearing Pan
- Prepare medium (quantities for 1 rearing pan)
- Combine wheat bran (500 g), wood shavings (200 g) and fish meal (115 g) in 10 L plastic dish pan and mix well.
- Add ammonium bicarbonate (50 g) to water (~25 °C, 1600 mL) and mix until dissolved.
- Add water solution to dry ingredients and mix to ensure no unwetted material. Level medium in pan but do not compress.
- Add stable fly eggs
- Make a shallow furrow in medium the length of the pan.
- Deposit 1 mL of stable fly eggs (~8,000 eggs) in furrow with egg pipette (Figure 2).
NOTE: Egg pipette made by cutting a plastic graduated pipette at desired point, inserting 100 mesh screen and gluing pipette back together. - Cover eggs with a thin layer of medium to prevent desiccation.
- Cover pan with pillow case, close with a rubber band and label. Place pans in larval room (23 ± 2 °C, 30–50% RH and 12:12 [L:D] h photoperiod).
NOTE: Larval development takes 10–14 days from the time of oviposition.
3. Pupal Processing, Larvae Move to The Edge of The Medium 7–9 Days After Oviposition and Pupariate by Days 13 or 14
- Scoop puparia, primarily located around the edges of the pan under the crust that forms on the surface of the medium, out and placed in a clean pan.
- Fill pan, with puparia, ½ to ¾ full of water. Break up clumps of puparia and medium. Wheat bran and protein components of the medium sink while puparia >1 day old float19.
- Remove floating materials, puparia and some medium components with a colander and wash through a series of sieves (#5, #7, #12, #20) to remove remaining medium. The #12 sieve collects the puparia and the #20 keeps solid waste from entering the drain. Wash puparia between sieves with a sink sprayer.
- Rinse puparia, in the #12 sieve, into a clean pan. Fill pan to ½ full of water. Pour floating puparia into a colander and allow excess water to drain.
- Transfer drained puparia to drying screen with fan and leave until dry (Figure 3).
4. Pupal Shelf, an Alternative Method for Collecting Puparia Is with a Pupal Shelf18
NOTE: The shelf is made from a piece of plastic cut from the end of a 10 L dish pan (10.2 cm tall x 10.2 cm wide x 31.9 cm long).
- Prepare medium as described in 2.1. Taper medium in pan from ~2.5 cm deep on one end to ~7.5 cm deep on the other.
- Place shelf on medium in the shallow end of the rearing pan and tape to prevent larvae from crawling alongside the shelf (Figure 4).
- Saturate sponge (~14.5 x 9.0 x 4.5 cm3, regenerated cellulose) and wrap it in a water soaked cloth (30.5 x 42 cm2, cotton) and place on the shelf about one half inch from the medium.
- Add eggs as described in 2.2 and proceed as before.
- Check pans daily to ensure that the sponge remains moist.
- Rinse puparia from the shelf, sponge and cloth into a clean dish pan and pour into colander 14 days after oviposition.
5. Quality Control
- Weigh all of the puparia produced in pan (total wt.).
- Isolate 100 puparia, weigh and placed in a 9 cm Petri dish.
- Count and sex adults ~5–8 days later to determine emergence rate and sex ratio. Store Petri dishes with emerged adults in a freezer for counting at a later date if needed. Flies are sexed by the shape of their eyes and width of the fronto-orbital plate20 or genitalia under low magnification.
- Record total weight, weight of 100 puparia, and number of emerged adult males and females.
6. Preparing Blood
- Collect fresh bovine blood from a local abattoir in 19 L buckets containing 70 g of sodium citrate tribasic dihydrate in 500 mL of water. Stir blood vigorously for 5–10 min and returned to the laboratory.
- Strain blood through a colander to remove clots and partitioned into 2 L containers, label with the date of collection and store in a freezer (-20 °C).
- Remove container from freezer and place in refrigerator 2 days before needed. Blood can be used for ~2 weeks once thawed and stored in the freezer for up to 1 year.
7. Adult Maintenance
- Prepare cages, line cage bottom with butcher paper to facilitate cleaning.
- Place 50 g of dry stable fly pupae (~3,500) in each cage. Adults eclose a few hours to a couple of days after pupal processing.
- Place a fresh blood soaked feminine napkin on top of each cage for feeding. Begin feeding within 24 h of adult eclosion and repeat daily until a day prior to oviposition (9–10 days after emergence).
- After oviposition (section 1), place cage in freezer for 4–8 h and then clean by removing the paper, rinsing frass from the sides and floor with hot water and scrubbing all surfaces with laundry detergent and chlorine bleach. Rinse cages with hot water and allow to dry.
Access restricted. Please log in or start a trial to view this content.
Wyniki
Larvae pupariate 10–14 days and adults emerge 14–16 days after oviposition. Generation time, egg to egg, is ~24 days. Rearing data for May 2013 to January 2017 with three different bulking agents and two colonies are presented in Figure 5. Cottonwood gave the best yield, 3867 ±1442 (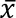 ± standard deviation) pupae weighing 12.5 ±1.6 mg with 74 ±19% eclosion producing...
± standard deviation) pupae weighing 12.5 ±1.6 mg with 74 ±19% eclosion producing...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
Stable flies can be found developing in a broad variety of substrates in nature and can be reared in many types of media in the laboratory. We have used wood shavings and vermiculite as bulking agents. Vermiculite worked well, but made separating puparia from rearing medium difficult and was expensive (~$0.60/pan). Possibly because of the added rigor of separating puparia from the medium, eclosion was also lower with vermiculite, 57% vs. 75% for wood shavings. Cottonwood shavings were comparable to vermiculite, but at ti...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We would like to thank Anthony Weinhold and the numerous students who have worked with us over the years for technical support as well as suggestions for improving our insect rearing procedures.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Diamalt | Premier Malt Products, Inc., Saddle Brook, NJ | 2540 | |
| CSMA Fly media | Purina Animal Nutrition, Arden Hills, MN | 5S6Z | |
| Thin Maxi Pad | The Tranzonic Co., Cleveland, OH, USA | 5001M | |
| Calf Manna | MannaPro, Chesterfield, MO, USA | Manna Pro | |
| Ammonium Bicarbonate | Spectrum Chemical Manufacturing Corp, Gardena, CA | A1125 | |
| Wheat bran, Coarse | Siemer Milling Company, Teutopolis, IL | ||
| Wood shavings | Tractor Supply Company, Brentwood, TN | 502770699 | |
| Fishmeal | Consumer Supply Distributing, North Sioux City, SD | F1550 | |
| Adult cages | All Aluminum Window Company, Lincoln, Ne | Custom | 45 × 45 × 45 cm, 18 × 16 mesh aluminum screen, stockinette access |
| 9 × 28 cm black cotton cloth | Robert Kaufman Fabrics, Los Angeles, CA | K040-114 | Egging cloth |
| 10 L plastic dish pans | Rubbermaid, Saratoga Springs, NY | FG2951ARWHT | Larval pans |
| Stockinette, Cotton, 12 inch x 25 yard roll | Tex-Care Medical Company, Burlington, NC | 91311-225 |
Odniesienia
- Cook, D. F., Dadour, I. R., Keals, N. J. Stable fly, house fly (Diptera: Muscidae), and other nuisance fly development in poultry litter associated with horticultural crop production. J. Econ. Entomol. 92 (6), 1352-1357 (1999).
- Dominghetti, T. F., de Barros, A. T., Soares, C. O., Cançado, P. H. Stomoxys calcitrans (Diptera: Muscidae) outbreaks: current situation and future outlook with emphasis on Brazil. Rev. Bras. Parasitol. Vet. 24 (4), 387-395 (2015).
- Solórzano, J. -A., Guilles, J., Bravo, O., Vargas, C., Gomez-Bonilla, Y., Bingham, G., Taylor, D. B. Biology and trapping of stable flies (Diptera: Muscidae) developing in pineapple residues (Ananas comosus) in Costa Rica. J. Insect Sci. 15 (1), 145(2015).
- Berry, I. L., Stage, D. A., Campbell, J. B. Populations and economic impacts of stable flies on cattle. Trans. Am. Soc. Agric. Eng. 26, 873-877 (1983).
- Taylor, D. B. Area-wide management of stable flies. Area-wide management of insect pests. Vreysen, J., Hendrichs, R., Cardoso Pereira, R. , in press (2017).
- Bishopp, F. C. The stable fly (Stomoxys calcitrans L.), an important live stock pest. J.Econ. Entomol. 6 (1), 112-126 (1913).
- Hogsette, J. A., Ruff, J. P., Jones, C. J. Stable fly biology and control in northwest Florida. J. Agric. Entomol. 4 (1), 1-11 (1987).
- Lysyk, T., Kalischuk-Tymensen, L., Selinger, L., Lancaster, R., Wever, L., Cheng, K. Rearing stable fly larvae (Diptera: Muscidae) on an egg yolk medium. J. Med. Entomol. 38, 382-388 (1999).
- Romero, A., Broce, A., Zurek, L. Role of bacteria in the oviposition behaviour and larval development of stable flies. Med. Vet. Entomol. 20, 115-121 (2006).
- Glaser, R. W. Rearing flies for experimental purposes with biological notes. J. Econ. Entomol. 17 (4), 486-496 (1924).
- Melvin, R. Physiological studies on the effect of flies and fly sprays on cattle. J. Econ. Entomol. 25 (6), 1151-1164 (1932).
- Doty, A. E. Convenient method of rearing the stable fly. J. Econ. Entomol. 30 (2), 367-369 (1937).
- Bridges, A. C., Spates, G. E. Larval medium for the stable fly Stomoxys calcitrans (L.). Southwest. Entomol. 8 (1), 6-10 (1983).
- Hogsette, J. A. New diets for production of house flies and stable flies (Diptera: Muscidae) in the laboratory. J. Econ. Entomol. 85 (6), 2291-2294 (1992).
- McGregor, W. S., Dreiss, J. M. Rearing stable flies in the laboratory. J. Econ. Entomol. 48 (3), 327-328 (1955).
- Goodhue, L. D., Cantrel, K. E. The use of vermiculite in medium for stable fly larvae. J. Econ. Entomol. 51 (2), 250(1958).
- Friesen, K., Berkebile, D. R., Zhu, J. J., Taylor, D. B. Augmenting laboratory rearing of stable fly (Diptera: Muscidae) larvae with ammoniacal salts. J. Insect Sci. 17 (1), 1-6 (2017).
- Berkebile, D. R., Weinhold, A. P., Taylor, D. B. A new method for collecting clean stable fly (Diptera:Muscidae) pupae of known age. Southwest. Entomol. 34 (4), 469-476 (2009).
- Champlain, R. A., Fisk, F. W., Dowdy, A. C. Some improvements in rearing stable flies. J. Econ. Entomol. 47 (5), 940-941 (1954).
- Zumpt, F. The stomoxyine biting flies of the world. , Gustav Fischer Verlag. Stuttgart. (1973).
- Wienhold, B. J., Taylor, D. B. Substrate properties of stable fly (Diptera: Muscidae) developmental sites associated with round bale hay feeding sites in eastern Nebraska. Environ. Entomol. 41 (2), 213-221 (2012).
- Friesen, K., Berkebile, D. R., Wienhold, B. J., Durso, L., Zhu, J., Taylor, D. B. Environmental parameters associated with stable fly (Diptera: Muscidae) development at hay feeding sites. Environ. Entomol. 45 (3), 570-576 (2016).
- Albuquerque, T. A., Zurek, L. Temporal changes in the bacterial community of animal feces and their correlation with stable fly oviposition, larval development, and adult fitness. Front. Microbiol. 5 (590), 1-9 (2014).
- Bailey, D. L., Whitfield, T. L., LaBrecque, G. C. Laboratory biology and techniques for mass producing the stable fly, Stomoxys calcitrans (L.) (Diptera: Muscidae). J. Med. Entomol. 12 (2), 189-193 (1975).
- Smith, J. P., Hall, R. D., Thomas, G. D. Field studies on mortality of the immature stages of the stable fly (Diptera: Muscidae). Environ. Entomol. 14 (6), 881-890 (1985).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone