Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Rapid and Specific Immunomagnetic Isolation of Mouse Primary Oligodendrocytes
W tym Artykule
Podsumowanie
We describe the immunomagnetic isolation of primary mouse oligodendrocytes, which allows the rapid and specific isolation of the cells for in vitro culture.
Streszczenie
The efficient and robust isolation and culture of primary oligodendrocytes (OLs) is a valuable tool for the in vitro study of the development of oligodendroglia as well as the biology of demyelinating diseases such as multiple sclerosis and Pelizaeus-Merzbacher-like disease (PMLD). Here, we present a simple and efficient selection method for the immunomagnetic isolation of stage three O4+ preoligodendrocytes cells from neonatal mice pups. Since immature OL constitute more than 80% of the rodent-brain white matter at postnatal day 7 (P7) this isolation method not only ensures high cellular yield, but also the specific isolation of OLs already committed to the oligodendroglial lineage, decreasing the possibility of isolating contaminating cells such as astrocytes and other cells from the mouse brain. This method is a modification of the techniques reported previously, and provides oligodendrocyte preparation purity above 80% in about 4 h.
Wprowadzenie
Oligodendrocytes (OLs) are the myelinating cells of the central nervous system (CNS)1. The isolation and culture of primary oligodendrocytes in a tightly regulated environment is a valuable tool for the in vitro study of the development of oligodendroglia as well as the biology of demyelinating diseases such as multiple sclerosis2. This requires an efficient and robust oligodendrocyte isolation and culture method3. In this study, we took advantage of the expression of a distinctive oligodendrocyte cell surface marker to implement a modified isolation technique that is rapid and specific.
Four distinct stages of oligodendrocyte maturation have been identified, each characterized by the expression of distinctive cell surface markers for each developmental stage (Figure 1). These cell surface markers can be recognized by specific antibodies4,5, and can be used to isolate OLs at specific stages. In the first stage, oligodendrocyte precursor cells (OPCs) have the capacity to proliferate, migrate, and specifically express platelet-derived growth factor receptor (PDGF-Rα)6, ganglioside A2B5, proteoglycan NG27,8, polysialic acid-neural cell adhesion molecule9 and fatty-acid-binding protein 7 (FABP7)10. OPCs have bipolar morphology with few short processes emanating from the opposing poles of the cell body, which is characteristic of neural precursor cells11.
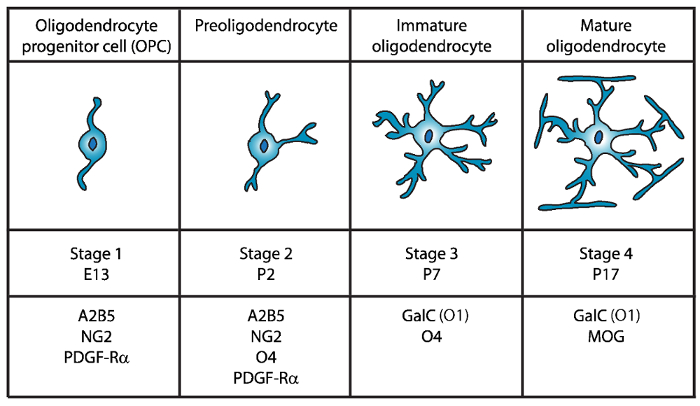
Figure 1: Expression of cell surface markers during the mouse oligodendrocyte development. OLs cell surface markers such as A2B5, GalC (O1), NG2, O4, and PDGF-Rα can be used to specifically isolate oligodendrocytes at specific developmental stage by using specific antibodies. Please click here to view a larger version of this figure.
In the second stage, OPCs give rise to preoligodendrocytes and express at the cell membrane not only OPC markers, but also the sulfatide (a sulfated galactolipid) recognized by the O4 antibody12,13, and the GPR17 protein14, which persists until the immature oligodendrocyte (OL) stage. At this stage, preoligodendrocytes extend multipolar short processes. Preoligodendrocytes are the major OL stage at postnatal day 2 (P2) in the cerebral white matter of both rat and mouse with a minor population of immature OLs15.
During the third stage, immature OLs continue to express O4, lose expression of A2B5 and NG2 markers and begin to express galactocerebroside C16. At this stage, OLs are committed to the oligodendroglial lineage and become post-mitotic cells with long ramified branches17,18. Immature OL constitute more than 80% of the rodent white matter at P7 and at this time the first MBP+ cells are observed15,19,20,21. Therefore, isolation of OLs at P7 could ensure high cellular yield.
In the final and fourth stage of OL development, mature OLs express myelinating proteins (myelin basic protein (MBP), proteolipid protein (PLP), myelin associated glycoprotein (MAG) and myelin oligodendrocyte glycoprotein (MOG)22,23,24,25,26. At this stage, mature OLs extend membranes that form compact enwrapping sheaths around the axons and are able to myelinate. This coincides with the observation that in rat and mouse brain, MBP+ cells become increasingly abundant at P1419,20,21.
Since the first isolation of oligodendrocyte by Fewster and colleagues in 196727, several methods for isolation of OLs from the rodent CNS have been implemented including immunopanning28,29,30, fluorescence-activated cell sorting (FACS) exploiting cell surface-specific antigens28,31, differential gradient centrifugation32,33,34,35 and a shaking method based on differential adherence of different CNS glia36,37. However, existing culture methods have limitations, particularly in terms of purity, yield and time required to perform the procedures38. Therefore, more efficient isolation methods for oligodendrocytes are required.
In this paper, we present a simple and efficient selection method for the immunomagnetic isolation of stage three O4+ preoligodendrocytes cells from neonatal mice pups. This method is a modification of the techniques reported by Emery et al.39 and Dincman et al.40 and provides an oligodendrocyte preparation purity above 80% in about 4 h.
Access restricted. Please log in or start a trial to view this content.
Protokół
The mice used in this study were cared for according to the guidelines of the SUNY Downstate Medical Center Division of Laboratory Animal Resources (DLAR) protocol number 15-10492.
NOTE: Primary oligodendrocytes were isolated from neonatal (P5-P7 wild-type C57Bl/6N) mice. At this stage, immature OLs constitute more than 80% of the rodent white matter ensuring high cellular yield. All buffer and reagent compositions are available at the end of the Table of Materials.
1. Coverslips Preparation
NOTE: Poly-D-lysine (PDL)/laminin coated coverslips should be prepared prior to OL isolation.
- Place #1 German glass coverslips into a 50-mL conical tube and add 35 mL of 70% EtOH to clean them.
- Close the tube, place it in a nutating mixer and incubate at room temperature for at least 30 min while gently rocking. Coverslips can be incubated overnight.
- Discard the 70% EtOH.
- Wash the coverslips 3 times with deionized water, remove the water every time with vacuum aspiration.
- Add 30-35 mL of PDL 50 µg/mL to the coverslips in the 50-mL tube, enough to cover the coverslips.
- Place the 50-mL tube containing the coverslips on a nutating mixer and incubate at room temperature for at least 30 min while rocking gently.
- Wash the coverslips 3 times with deionized water, removing the water every time with vacuum aspiration.
- Transfer the coverslips into 60-mm Petri dishes containing deionized water.
- Use a P200 pipette tip to arrange the coverslips as a layer covering the entire surface of the Petri dish.
- Carefully, remove the water from the dishes with vacuum aspiration.
- Remove the excess of water around the coverslips and let them dry overnight with lid open and under UV light in the hood.
NOTE: PDL coated glass coverslips can be stored at 4 °C in sterile Petri dishes for up to three months. - Place the coverslips into 24-well plates, one per well, using fine forceps.
- Move the coverslips to the center of the well making sure the edges do not touch the wall of the well.
- Add 100 µL of 10 µg/mL laminin diluted in B27NBMA (see the Table of Materials) starting in the center of each coverslip and moving in a circular fashion towards the edges to cover all the area.
NOTE: Since laminin is involved in OL maintenance and promotes differentiation into mature OLs, it is an important OL survival factor for in vitro culture41,42,43,44. - Incubate the plate in a 37 °C incubator at 5% CO2 for at least 1 h or until the OLs are ready for plating.
2. Mouse Brain Cortex Dissociation
- Sacrifice postnatal 5-7-day old C57Bl/6N mouse pups by quick decapitation with scissors previously cleaned in 70% ethanol.
NOTE: Cerebral cortices from 5-6 neonatal mice pups were used for each preparation. On average, one mouse brain yields 1-1.5 x 106 OLs. - Cut the scalp skin along the midline with small dissecting scissors and retract to expose the skull.
- Cut the skull carefully along the midline starting from the opening in the back of the skull towards the frontal area, lifting up with the scissors to avoid damaging the brain. Then, using the opening in the back of the skull cut toward each eye socket along the base of the skull.
- Use fine forceps to gently tease the cortices away from the midbrain and transfer them to a 60-mm tissue culture dish containing 7 mL of B27NBMA. Repeat steps 2.2 through 2.4 for each brain, pooling the dissected cortices.
- Transfer the cortices to a new 60-mm tissue culture dish containing 5 mL of dissociation buffer (B27NBMA, 20-30 U/mL Papain, and 2,500 U DNase I).
- Dice the cortices into small pieces of about 1 mm3 using a #15 scalpel blade and incubate for 20 min in a 37 °C, 5% CO2 incubator.
- Add 1 mL of bovine growth serum (BGS) to stop the enzymatic reaction.
- Transfer the cortices along with the dissociation media to a 15-mL conical tube using a 10 mL serological pipet.
- Gently begin to dissociate the brain tissue by slowly pipetting up and down 6-8 times using a 10-mL serological pipet, using care to minimize bubbles.
- Allow the tissue chunks to settle for 2-3 min and transfer the supernatant to a fresh tube.
- Add 3 mL of B27NBMA containing 10% BGS/2500 U DNase I to the tissue pellet.
- Use a 5 mL serological pipette to gently dissociate the brain tissue while pipetting up and down 6-8 times. Be careful to minimize bubbles.
- Allow the tissue chunks to settle for 2-3 min.
- Transfer the supernatant to a fresh tube. Add 3 mL of B27NBMA containing 10% BGS/DNase I to the tissue pellet.
- Gently dissociate the brain tissue using a P1000 pipet tip, while pipetting the mix of media and brain tissue up and down. Repeat Steps 2.13 and 2.14 until no large chunks of tissue remain or until B27NBMA containing 10% BGS/DNase I is exhausted, whichever comes first. Be careful to minimize bubbles.
- Pool cell suspension with previous supernatants.
- Place 70-µm cell strainer in a 50-mL tube. Using a 10-mL serological pipet, pass the pooled cell suspension gently over the 70-µm cell strainer.
- Wash the cell strainer by adding 1 mL of B27NBMA containing 10% BGS/DNase I.
- Discard the cell strainer. Bring the volume to 30-mL with B27NBMA containing 10% BGS/DNase I.
- Transfer the cell suspension to two 15-mL conical tubes. Centrifuge the cell suspension for 10 min at 200 x g.
- Remove the supernatant without leaving the cells exposed to the air. The supernatant will be cloudy due to cell debris. Add 3 mL of B27NBMA containing 10% BGS to the pellet.
- Carefully dissociate the cell pellet using a P1000 pipet. Bring the volume to 15 mL with B27NBMA containing 10% BGS.
- Pass the cell suspension over a fresh 40 µm cell strainer.
- Wash the cell strainer by adding 1mL of B27NBMA containing 10% BGS. Discard the cell strainer.
- Bring the volume to 30 mL with B27NBMA containing 10% BGS.
- Transfer the cell suspension to 2 x 15-mL conical tubes. Centrifuge the cell suspension for 10 min at 200 x g.
- Remove most of the supernatant without leaving the cells exposed to the air. Resuspend the cell pellets in 5 mL of ice cold magnetic cell sorting (MCS) buffer.
3. Determination of Cell Count and Viability
- Dilute 100 µL of the cell suspension with 400 µL of Trypan Blue Solution in a 1.5-mL microcentrifuge tube to achieve a 1:5 dilution in 0.4% (w/v).
- Center a cover glass over a hemocytometer chamber and fill the two chambers with 10 µL of the cell dilution using a P10 pipette and avoiding overfill. The solution will pass under the cover glass by capillary action.
- Place the hemocytometer on the microscope stage and adjust focus using 40X magnification.
NOTE: Cell counts are recorded using a hand-held counter in each of five squares (four corners and one center). Only non-viable cells absorb the dye and appear blue, while live and healthy cells appear round and refractive and do not absorb the blue-colored dye, allowing for determination of the number of viable and total cells per milliliter.
4. Isolation of O4+ Oligodendrocytes
- Centrifuge the cell suspension at 200 x g for 10 min.
- Carefully discard the supernatant by using vacuum aspiration, avoiding exposure of the cells to the air.
- Resuspend the pellet in 90 µL of MCS buffer per 1 x 107 total cells followed by the addition of 10 µL of anti-O4 beads per 1 x 107 cells.
- Mix the cell suspension and beads by gently flicking the 15-mL conical tube with the finger 4-5 times.
- Incubate the mix for 15 min at 4 °C, flicking the 15-mL conical tube with the finger 4-5 timesevery 5 min.
- Wash the mix by gently adding 2 mL of MCS buffer per 1 x 107 cells to the tube. Centrifuge the mix at 200 x g for 10 min.
- Discard the supernatant by carefully using vacuum aspiration, avoiding exposure of the cells to the air. Resuspend the cell pellet in 500 µL of MCS buffer for every 1 x 107 cells.
- Attach a magnetic separator to a magnetic separator stand. Attach a selection column to the magnetic separator and place a 40 µm cell strainer on top of the column. Place two 15-mL or one 50-mL conical tube below the separation column to collect the flow through.
- Pre-rinse the 40-µm cell strainer and separation column with 3 mL of MCS buffer and let the buffer run through the column without letting it dry.
- Add the mix of cell suspension and beads to the cell strainer and into the selection column.
- Wash the 40 µm cell strainer with 1 mL of MCS buffer.
- Discard the cell strainer and let the mix of cells, beads and buffer run through the column without letting it dry.
- Wash the separation column 3 times with 3 mL of MCS buffer and 1 time with OL proliferation media.
- Remove the separation column from the magnetic separator, quickly place it into a 15-mL conical tube, and immediately add 5 mL of OL proliferation media.
- Place a plunger on top of the column and firmly push to flush out the labeled cells into the 15-mL conical tube.
- Remove 100 µL of cell suspension to determine cell count and viability using Trypan Blue and hemocytometer as indicated in section 3.
NOTE: Cell viability above 80% is considered acceptable to proceed with the culture of OLs, but viability greater than 90% is optimal.
5. Plating of Isolated O4+ Oligodendrocytes
- Dilute the cell suspension to the desire plating density (5 x 105 cells per coverslip) using OL proliferation media.
NOTE: OL seeding density is very important, so appropriate cell density should be confirmed using a tissue culture microscope prior to proceeding. Seeding density below 10,000 cells/coverslips may lead to poor cell survival. At plating density at 10,000 to 25,000, OL morphological differentiation is slow45. We plate OLs at a seeding density of at least 50,000 cells/coverslips to ensure cell survival. - Move the 24-well plates containing coverslips coated with laminin from incubator to the tissue culture hood.
- Remove laminin from each coverslip and replace it with 100 µL of OL suspension.
- Incubate OLs in the 37 °C/5% CO2 incubator for a maximum of 45 min to promote OL adhesion to the coverslips.
- After incubation, remove the 24-well plates from the incubator. Flood each well of the 24-well plate with 500 µL of OL proliferation media.
- Place the OLs back in the 37 °C/5% CO2 incubator for additional 24 h.
- 24 h after plating of OLs, remove proliferation media and substitute with OL differentiation media to induce differentiation.
- Place the OLs back in the incubator and do not remove until the cells are ready for fixation.
NOTE: Changes in pH are detrimental for OLs and decrease cell viability, therefore unnecessary removal of OLs cultures from the incubator should be avoided.
6. Immunofluorescence Staining
- For the detection of cell surface markers (NG2, O1 and O4) remove the media from the wells.
- Add 250 µL of mouse hybridoma supernatants containing primary antibodies against O1 (undiluted)13 and O4 (undiluted)13 and rabbit polyclonal against NG2 (1:150 diluted in B27NBMA).
NOTE: If commercially available anti-O1 and anti-O4 antibodies are to be used, it is advisable to utilize dilutions suggested by the manufacturer. - Incubate the cells with primary antibody for 45 min maximum in a 37 °C/5% CO2 incubator.
- Wash twice with 0.05% Tween-20/1X PBS. Fix the cells with 4% paraformaldehyde (4% PFA) for 10 min at room temperature.
- Wash three times for 5 min each with 0.05% Tween-20/1X PBS.
- Dilute Alexa 488-conjugated goat anti-mouse IgM or anti-rabbit IgG secondary antibody (1:400) in B27NBMA.
- Remove the 0.05% Tween-20/1X PBS from the coverslips. Incubate the cells with secondary antibody for 1 h at room temperature in the dark.
- Wash three times for 5 min each with 0.05% Tween-20/1X PBS.
- For double staining for the detection of internal markers (GFAP), remove the 0.05% Tween-20/1X PBS and block/permeabilize cells with block/permeabilization buffer for 15 min at room temperature. Otherwise proceed to step 6.17.
- Remove block/permeabilization buffer and add 250 µL chicken anti-GFAP (1:100) diluted in block/permeabilization buffer.
- Incubate the cells with anti-GFAP for 1 h at room temperature. Wash three times for 5 min each with 0.05% Tween-20/1X PBS.
- Dilute Alexa 594-conjugated goat anti-chicken IgG secondary antibody (1:400) in B27NBMA.
- Remove the 0.05% Tween-20/1X PBS from the coverslips. Incubate the cells with secondary antibody for 1 h at room temperature in the dark.
- Wash three times for 5 min each with 0.05% Tween-20/1X PBS.
- Counterstain with 4',6-diamidino-2-phenylindole (DAPI) and mount the cells with antifade mounting media containing DAPI.
- Let the coverslips cure overnight in the dark at room temperature.
- Image the stained cells with an epifluorescence microscope.
NOTE: In this protocol, the cells were imaged at 40X magnification using a uniform exposure time.
Access restricted. Please log in or start a trial to view this content.
Wyniki
The purpose of this study was to establish an improved isolation method for O4+ primary mouse oligodendrocytes requiring the least possible manipulation of the target cells. The entire procedure from euthanasia of the pups to plating of the cells in coverslips takes about 4 h and data shown here represent three independent experiments. After tissue dissociation, an average of 4.3 ± 0.46 x 107 cells were isolated for each independent experiment, with a viability of 9...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
In this communication, we present a method for the efficient isolation of highly purified immature mouse oligodendrocyte cultures. Compared to previously published protocols39,40, this method yielded a higher purity with a much lower level of GFAP-positive astrocytes and a very low percentage of other non-characterized cells. It is important to point out that these are immature OLs already committed to the oligodendroglial lineage. Thus, these cells would not be ...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have no disclosures.
Podziękowania
This study was supported by grants from the National Multiple Sclerosis Society (RG4591A1/2) and the National Institutes of Health (R03NS06740402). The authors thank Dr. Ivan Hernandez and his lab members for providing laboratory space, equipment and advice.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| 10ml serological pipets | Fisher Scientific | 13-676-10J | |
| 10ml syringe Luer-Loc tip | BD, Becton Dickinson | 309604 | |
| 15ml conical tubes | Falcon | 352097 | |
| 24-well tissue culture plates | Falcon | 353935 | |
| 40µm cell strainer | Fisher Scientific | 22368547 | |
| 50ml conical tubes | Falcon | 352098 | |
| 5ml serological pipets | Fisher Scientific | 13-676-10H | |
| 60mm tissue culture plates | Falcon | 353002 | |
| 70µm cell strainer | Fisher Scientific | 22363548 | |
| Alexa Fluor 488 goat anti-mouse IgG (H+L) secondary antibody | Invitrogen | A11001 | |
| Alexa Fluor 488 goat anti-rabbit IgM (H+L) secondary antibody | Invitrogen | A21042 | |
| Alexa Fluor 488 goat anti-rabbit IgM (H+L) secondary antibody | Invitrogen | A11008 | |
| Alexa Fluor 594 goat anti-chicken IgG (H+L) secondary antibody | Invitrogen | A11042 | |
| Anti-O4 beads- Anti-O4MicroBeads | Miltenyi Biotec | 130-094-543 | |
| Apo-Transferrin human | Sigma | T1147 | |
| Autofil complete bottle top filter assembly, 0.22um filter, 250ml | USA Scientific | 6032-1101 | |
| Autofil complete bottle top filter assembly, 0.22um filter, 250ml | USA Scientific | 6032-1102 | |
| B27 Supplement | Invitrogen | 17504-044 | |
| Boric acid | Sigma | B7660 | |
| Bovine Growth Serum (BGS) | GE Healthcare Life Sciences | SH30541.03 | |
| BSA | Fisher Scientific | BP-1600-100 | |
| CNTF | Peprotech | 450-50 | |
| d-Biotin | Sigma | B4639 | |
| Desoxyribonuclease I (DNAse I) | Worthington | LS002007 | |
| EDTA | Fisher Scientific | S311 | |
| Epifluorescence microscope with an Olympus DP70 camera | Olympus | Bx51 | |
| Feather disposable scalpels | Andwin Scientific | EF7281C | |
| Forskolin | Sigma | F6886 | |
| German glass coverslips, #1 thickness, 12mm diameter round | NeuVitro | GG-12-oz | |
| GFAP antibody | Aves | GFAP | |
| Glucose | Fisher Scientific | D16-1 | |
| GlutaMAX | Invitrogen | 35050-61 | |
| Insulin | Invitrogen | 12585-014 | |
| Magnetic separator stand - MACS multistand | Miltenyi Biotec | 130-042-303 | |
| Magnetic separator-MiniMACS separator | Miltenyi Biotec | 130-042-302 | |
| Millex PES 0.22µm filter unit | Millipore | SLG033RS | |
| Mounting media- Prolong Gold with DAPI | Thermo Fisher | P36930 | |
| N-acetyl-cysteine (NAC) | Sigma | A8199 | |
| Natural mouse laminin | Invitrogen | 23017-015 | |
| Neurobasal Medium A | Invitrogen | 10888-022 | |
| Neurotrophin-3 (NT-3) | Peprotech | 450-03 | |
| NG2 antibody | Millipore | AB5320 | |
| Papain | Worthington | LS003126 | |
| PBS without Ca2+ and Mg2+ | Sigma | D5652 | |
| PDGF | Peprotech | 100-13A | |
| Petri dishes | Falcon | 351029 | |
| Poly-D-Lysine | Sigma | P6407 | |
| Primocin | Invivogen | ant-pm-2 | |
| Progesterone | Sigma | P8783 | |
| Putrescine | Sigma | P5780 | |
| Selection column-LS columns | Miltenyi Biotec | 130-042-401 | |
| Sodium Selenite | Sigma | S5261 | |
| Trace elements B | Corning | 25-000-CI | |
| Triiodothyronine (T3) | Sigma | T6397 | |
| Triton-X | Sigma | T8787 | |
| Trypan Blue Solution | Corning | 25-900-CI | |
| Tween 20 | Sigma | P1379 | |
| B27NBMA | 487.75 mL Neurobasal Medium A; 10 mL B27 Supplement; 1 mL Primocin; 1.25 mL Glutamax; Filter sterilize and store at 4 °C until use. | ||
| B27NBMA + 10% BGS | 27 mL B27NBMA; 3 mL Bovine growth serum | ||
| CNTF solution stock (10 µg/ml; 1000X) | Order from Peprotech (450-50). Make up at 0.1 to 1 mg/ml according to Manufacturer’s instruction (may vary from lot to lot) in buffer (e.g. DPBS + 0.2% BSA). Store at -80 °C. Working solution (10 µg/ml, 1000X) 1. Make on 0.2% BSA (Fisher scientific BP-1600-100) in DPBS solution and filter sterilize. 2. Dilute master stock aliquot to 10µg/ml in sterile, chilled 0.2% BSA/DPBS. 3. Aliquot (20µl/tube) and snap freeze in liquid nitrogen. 4. Store aliquots at -80 °C. | ||
| d-Biotin stock solution (50 µg/ml; 5000X) | Resuspend d-Biotin (Sigma-B4639) in double-distilled H2O at 50 µg/ml (e.g. 2.5 mg in 50 ml of ddH2O). Resuspension might take fair amount of agitation/vortexing, or mild warming briefly at 37°C. If the d-Biotin still will not solubilize, it is fine to make up a less concentrated (e.g. 10µg/ml), and to add a higher volume to the B27NBMA (1/1000), instead of 1/5000). Store at 4°C. | ||
| DNase I stock solution | 1. Dissolve at 12,500 U Deoxyribonuclease I / ml in HBSS chilled on ice. 2. Filter sterilize on ice 3. Aliquot at 200 µl and freeze overnight at -20°C. 4. Store aliquots at -20 to -30°C. | ||
| Dulbecco’s Phosphate Buffered Saline (w/o Ca2+ and Mg2+) | Dissolve pouch in 1 Liter of water to yield 1 liter of medium at 9.6 grams of powder per liter of medium. Store at 2-8 °C. | ||
| Forskolin stock solution (4.2 mg/ml; 1000X) | Add 1 ml of sterile DMSO to 50 mg Forskolin in bottle (Sigma-F6886) and pipette until resuspended. Transfer to a 15 ml centrifuge tube and add 11 ml of sterile DMSO to bring to 4.2 mg/ml. Aliquot (e.g. 20 µl) and store at -20°C. | ||
| Hank’s balanced salts (HBSS) (Sigma | 1. Measure 900 ml of water (temperature 15-20 °C) in a cylinder and stir gently. 2. Add the power and stir until dissolved. 3. Rinse original package with a small amount of water to remove all traces of the powder. 4. Add to the solution in step 2. 5. Add 0.35 gr of sodium bicarbonate (7.5% w/v) for each liter of final volume. 6. Keep stirring until dissolved. 7. Adjust the pH of the buffer while stirring to 0.1-0.3 units below pH= 7.4 since it may rise during filtration. The use of 1N HCl or 1N NaOH is recommended to adjust the pH. 8. Add additional water to bring the final volume to 1L. 9. Sterilize by filtration using a membrane with a porosity of 0.22 microns. 10. Store at 2-8 °C. | ||
| Insulin stock solution (4000 µg/ml) | Thaw the bottle and aliquot 25 µl per microcentrifuge tube and store at -20°C. | ||
| Laminin solution | Slowly thaw laminin in the cold (2°C to 8°C) to avoid gel formation. Then, aliquot into polypropylene tubes. Store at 5° C to -20° C in aliquots (e.g. 20 µl) and do not freeze/thaw repeatedly. Laminin may be stored at these temperatures for up to six months. | ||
| Magnetic Cell Sorting (MCS) Buffer | Prepare the solution containing phosphate-buffered saline (PBS), pH 7.2, and 0.5% bovine serum albumin (BSA), 0.5 mM EDTA, 5µg/ml Insulin, 1 g/L Glucose. Sterilize and degas by filtration the buffer by passing it through a 0.22 µm Millex filter. Store the buffer at 4°C until use | ||
| N-Acetyl-L-cysteine (NAC) stock solution (5mg/ml; 1000X) | Dissolve N-Acetyl-L-cysteine (Sigma-A8199) at 5 mg/ml in DMEM (e.g. 50 mg NAC in 10 ml B27NBMA). Filter sterilize and aliquot (e.g. 20 µl). Store at -20°C. | ||
| NT3 stock solution (1 µg/ml; 1000X) | Master stock: Order from Peprotech (450-03). Make up at 0.1 to 1 mg/ml according to manufacturer’s instructions (may vary from lot to lot), in buffer (e.g. DPBS + 0.2% BSA). Store at -80°C. Working stock (1µg/ml; 1000X): 1. Make on 0.2% BSA in DPBS solution and filter sterilize. 2. Dilute master stock aliquot to 1 µg/ml in sterile, chilled 0.2% BSA/DPBS. 3. Aliquot (e.g. 20µl/tube) and snap freeze in liquid nitrogen. 4. Store aliquots at -80°C. | ||
| PDGF stock solution (10 µg/ml; 1000X) | Master stock: Order from Peprotech (100-13A). Make up at 0.1 to 1 mg/ml according to manufacturer’s instructions (may vary from lot to lot) in buffer (e.g. DPBS) + 0.2% BSA). Store at -80°C. Working stock (1µg/ml; 1000X): 1. Make on 0.2% BSA in DPBS solution and filter sterilize. 2. Dilute master stock aliquot to 1µg/ml in sterile, chilled 0.2% BSA/DPBS. 3. Aliquot (e.g. 20µl/tube) and snap freeze in liquid nitrogen. 4. Store aliquots at -80°C. | ||
| Poly-D-lysine (1mg/ml; 100X) | Resuspend poly-D-lysine, molecular weight 70-150 kD (Sigma P6407) at 0.5mg/ml in 0.15M boric acid pH 8.4 (e.g. 50mg in 50ml borate buffer). Filter sterilize and aliquot (e.g. 100µl/tube). Store at -20°C. Prior to use, dilute the 100X stock (1mg/ml) to 50 µg/ml in sterile water. | ||
| Oligodendrocyte proliferation media | see Supplementary Table 1 | ||
| Oligodendrocyte differentiation media | see Supplementary Table 1 | ||
| Sato supplement (100X) | see Supplementary Table 1 | ||
| References: the list of reagents and recipes were adopted from the protocols previously described by Emery et. al. 2013 (Emery, B. & Dugas, J. C. Purification of oligodendrocyte lineage cells from mouse cortices by immunopanning. Cold Spring Harb Protoc. 2013 (9), 854-868, doi:10.1101/pdb.prot073973, (2013)) and Dincman et. al. (Dincman, T. A., Beare, J. E., Ohri, S. S. & Whittemore, S. R. Isolation of cortical mouse oligodendrocyte precursor cells. J Neurosci Methods. 209 (1), 219-226, doi:10.1016/j.jneumeth.2012.06.017, (2012)) |
Odniesienia
- Emery, B. Regulation of oligodendrocyte differentiation and myelination. Science. 330 (6005), 779-782 (2010).
- Yang, Z., Watanabe, M., Nishiyama, A. Optimization of oligodendrocyte progenitor cell culture method for enhanced survival. J Neurosci Methods. 149 (1), 50-56 (2005).
- Niu, J., et al. An efficient and economical culture approach for the enrichment of purified oligodendrocyte progenitor cells. J Neurosci Methods. 209 (1), 241-249 (2012).
- Zhang, S. C. Defining glial cells during CNS development. Nat Rev Neurosci. 2 (11), 840-843 (2001).
- Pfeiffer, S. E., Warrington, A. E., Bansal, R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 3 (6), 191-197 (1993).
- Hart, I. K., Richardson, W. D., Heldin, C. H., Westermark, B., Raff, M. C. PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (O-2A) cell lineage. Development. 105 (3), 595-603 (1989).
- Nishiyama, A., Lin, X. H., Giese, N., Heldin, C. H., Stallcup, W. B. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 43 (3), 315-330 (1996).
- Pringle, N. P., Mudhar, H. S., Collarini, E. J., Richardson, W. D. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 115 (2), 535-551 (1992).
- Grinspan, J. B., Franceschini, B. Platelet-derived growth factor is a survival factor for PSA-NCAM+ oligodendrocyte pre-progenitor cells. J Neurosci Res. 41 (4), 540-551 (1995).
- Sharifi, K., et al. Differential expression and regulatory roles of FABP5 and FABP7 in oligodendrocyte lineage cells. Cell Tissue Res. 354 (3), 683-695 (2013).
- Chittajallu, R., Aguirre, A., Gallo, V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 561 (Pt 1), 109-122 (2004).
- Bansal, R., Warrington, A. E., Gard, A. L., Ranscht, B., Pfeiffer, S. E. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res. 24 (4), 548-557 (1989).
- Sommer, I., Schachner, M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 83 (2), 311-327 (1981).
- Boda, E., et al. The GPR17 receptor in NG2 expressing cells: focus on in vivo cell maturation and participation in acute trauma and chronic damage. Glia. 59 (12), 1958-1973 (2011).
- Dean, J. M., et al. Strain-specific differences in perinatal rodent oligodendrocyte lineage progression and its correlation with human. Dev Neurosci. 33 (3-4), 251-260 (2011).
- Yu, W. P., Collarini, E. J., Pringle, N. P., Richardson, W. D. Embryonic expression of myelin genes: evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron. 12 (6), 1353-1362 (1994).
- Armstrong, R. C., Dorn, H. H., Kufta, C. V., Friedman, E., Dubois-Dalcq, M. E. Pre-oligodendrocytes from adult human CNS. J Neurosci. 12 (4), 1538-1547 (1992).
- Gard, A. L., Pfeiffer, S. E. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 106 (1), 119-132 (1989).
- Bjelke, B., Seiger, A. Morphological distribution of MBP-like immunoreactivity in the brain during development. Int J Dev Neurosci. 7 (2), 145-164 (1989).
- Hardy, R. J., Friedrich, V. L. Jr Progressive remodeling of the oligodendrocyte process arbor during myelinogenesis. Dev Neurosci. 18 (4), 243-254 (1996).
- Hartman, B. K., Agrawal, H. C., Kalmbach, S., Shearer, W. T. A comparative study of the immunohistochemical localization of basic protein to myelin and oligodendrocytes in rat and chicken brain. J Comp Neurol. 188 (2), 273-290 (1979).
- Wei, Q., Miskimins, W. K., Miskimins, R. Stage-specific expression of myelin basic protein in oligodendrocytes involves Nkx2.2-mediated repression that is relieved by the Sp1 transcription factor. J Biol Chem. 280 (16), 16284-16294 (2005).
- Stolt, C. C., et al. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 16 (2), 165-170 (2002).
- Emery, B., et al. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 138 (1), 172-185 (2009).
- Reynolds, R., Wilkin, G. P. Development of macroglial cells in rat cerebellum. II. An in situ immunohistochemical study of oligodendroglial lineage from precursor to mature myelinating cell. Development. 102 (2), 409-425 (1988).
- Scolding, N. J., et al. Myelin-oligodendrocyte glycoprotein (MOG) is a surface marker of oligodendrocyte maturation. J Neuroimmunol. 22 (3), 169-176 (1989).
- Fewster, M. E., Scheibel, A. B., Mead, J. F. The preparation of isolated glial cells from rat and bovine white matter. Brain Res. 6 (3), 401-408 (1967).
- Gard, A. L., Williams, W. C. 2nd, Burrell, M. R. Oligodendroblasts distinguished from O-2A glial progenitors by surface phenotype (O4+GalC-) and response to cytokines using signal transducer LIFR beta. Dev Biol. 167 (2), 596-608 (1995).
- Gard, A. L., Pfeiffer, S. E. Glial cell mitogens bFGF and PDGF differentially regulate development of O4+GalC- oligodendrocyte progenitors. Dev Biol. 159 (2), 618-630 (1993).
- Barres, B. A., Raff, M. C. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 361 (6409), 258-260 (1993).
- Behar, T., McMorris, F. A., Novotny, E. A., Barker, J. L., Dubois-Dalcq, M. Growth and differentiation properties of O-2A progenitors purified from rat cerebral hemispheres. J Neurosci Res. 21 (2-4), 168-180 (1988).
- Vitry, S., Avellana-Adalid, V., Lachapelle, F., Baron-Van Evercooren, A. Migration and multipotentiality of PSA-NCAM+ neural precursors transplanted in the developing brain. Mol Cell Neurosci. 17 (6), 983-1000 (2001).
- Duncan, I. D., Paino, C., Archer, D. R., Wood, P. M. Functional capacities of transplanted cell-sorted adult oligodendrocytes. Dev Neurosci. 14 (2), 114-122 (1992).
- Goldman, J. E., Geier, S. S., Hirano, M. Differentiation of astrocytes and oligodendrocytes from germinal matrix cells in primary culture. J Neurosci. 6 (1), 52-60 (1986).
- Althaus, H. H., Montz, H., Neuhoff, V., Schwartz, P. Isolation and cultivation of mature oligodendroglial cells. Naturwissenschaften. 71 (6), 309-315 (1984).
- McCarthy, K. D., de Vellis, J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 85 (3), 890-902 (1980).
- Szuchet, S., Yim, S. H. Characterization of a subset of oligodendrocytes separated on the basis of selective adherence properties. J Neurosci Res. 11 (2), 131-144 (1984).
- Chew, L. J., DeBoy, C. A., Senatorov, V. V. Jr Finding degrees of separation: experimental approaches for astroglial and oligodendroglial cell isolation and genetic targeting. J Neurosci Methods. 236, 125-147 (2014).
- Emery, B., Dugas, J. C. Purification of oligodendrocyte lineage cells from mouse cortices by immunopanning. Cold Spring Harb Protoc. 2013 (9), 854-868 (2013).
- Dincman, T. A., Beare, J. E., Ohri, S. S., Whittemore, S. R. Isolation of cortical mouse oligodendrocyte precursor cells. J Neurosci Methods. 209 (1), 219-226 (2012).
- Buttery, P. C., ffrench-Constant, C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 14 (3), 199-212 (1999).
- Chun, S. J., Rasband, M. N., Sidman, R. L., Habib, A. A., Vartanian, T. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol. 163 (2), 397-408 (2003).
- Colognato, H., Ramachandrappa, S., Olsen, I. M., ffrench-Constant, C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 167 (2), 365-375 (2004).
- ffrench-Constant, C., Colognato, H. Integrins: versatile integrators of extracellular signals. Trends Cell Biol. 14 (12), 678-686 (2004).
- Oh, L. Y., Yong, V. W. Astrocytes promote process outgrowth by adult human oligodendrocytes in vitro through interaction between bFGF and astrocyte extracellular matrix. Glia. 17 (3), 237-253 (1996).
- Besnard, F., Perraud, F., Sensenbrenner, M., Labourdette, G. Effects of acidic and basic fibroblast growth factors on proliferation and maturation of cultured rat oligodendrocytes. Int J Dev Neurosci. 7 (4), 401-409 (1989).
- Armstrong, R., Friedrich, V. L., Holmes, K. V., Dubois-Dalcq, M. In vitro analysis of the oligodendrocyte lineage in mice during demyelination and remyelination. J Cell Biol. 111 (3), 1183-1195 (1990).
- Grinspan, J. B., Stern, J. L., Franceschini, B., Pleasure, D. Trophic effects of basic fibroblast growth factor (bFGF) on differentiated oligodendroglia: a mechanism for regeneration of the oligodendroglial lineage. J Neurosci Res. 36 (6), 672-680 (1993).
- Mason, J. L., Goldman, J. E. A2B5+ and O4+ Cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Mol Cell Neurosci. 20 (1), 30-42 (2002).
- Schildge, S., Bohrer, C., Beck, K., Schachtrup, C. Isolation and culture of mouse cortical astrocytes. J Vis Exp. (71), (2013).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone