Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Förster Resonance Energy Transfer Measurements in Living Plant Cells
W tym Artykule
Podsumowanie
A protocol is provided for setting up a standard confocal laser-scanning microscope for in vivo Förster resonance energy transfer measurements, followed by data evaluation.
Streszczenie
Sensitized emission-based Förster resonance energy transfer (FRET) experiments are easily done but depend on the microscopic setup. Confocal laser scanning microscopes have become a workhorse for biologists. Commercial systems offer high flexibility in laser power adjustment and detector sensitivity and often combine different detectors to obtain the perfect image. However, the comparison of intensity-based data from different experiments and setups is often impossible due to this flexibility. Biologist-friendly procedures are of advantage and allow for simple and reliable adjustment of laser and detector settings.
Furthermore, as FRET experiments in living cells are affected by the variability in protein expression and donor-acceptor ratios, protein expression levels must be considered for data evaluation. Described here is a simple protocol for reliable and reproducible FRET measurements, including routines for the estimation of protein expression and adjustment of laser intensity and detector settings. Data evaluation will be performed by calibration with a fluorophore fusion of known FRET efficiency. To improve simplicity, correction factors have been compared that have been obtained in cells and by measuring recombinant fluorescent proteins.
Wprowadzenie
Förster resonance energy transfer ((F)RET) is typically observed by fluorescence spectroscopy, although the process itself is not limited to occur between fluorophores. The underlying dipole-dipole coupling simply requires a light-emitting donor molecule and a light-absorbing acceptor. This is derived from the required spectral overlap integral J of the normalized donor emission and acceptor absorbance spectra1. However, because RET competes with fluorescence, the energy transfer becomes measurable by alterations in fluorescence emission: RET induces donor quenching and sensitized acceptor emission.
Fluorophore-based RET has been termed fluorescence resonance energy transfer (FRET) to separate it from bioluminescence resonance energy transfer (BRET). RET depends strongly on the distance between donor and acceptor, which is widely in the range of 0.5-10 nm2 and thus, in the same range as the dimensions of proteins and their complexes. Second, RET depends on the dipole-dipole orientation kappa squared. Combined with the fact that rotational freedom of protein-bound fluorophores can be neglected due to the molecular weight and the slow rotational relaxation, RET allows for the analysis of conformational alterations3.
The so-called Förster radius is based on the spectral overlap integral and the wavelength range of the overlap, so that red light-absorbing chromophores result in longer Förster radii than blue light-absorbing dyes. As the dynamic range of FRET measurements is limited by 0.5 × R0 and 1.5 × R0, the FRET pair ECFP-EYFP has a dynamic range of 2.5-7.3 nm due to its R0 of 4.9 nm4.
The brightness of a fluorophore is given by the product of its molar extinction coefficient and its quantum yield. For FRET measurements, it is advantageous to choose fluorophores of nearly similar brightness. This enhances the detection of donor quenching and sensitized acceptor emission. It also favors the calibration of the microscopy system. Looking at the frequently used FRET pairs of cyan and fluorescent proteins, the lower brightness of the cyan fluorescent proteins becomes obvious (Figure 1A).
However, the lifetime of the acceptor must be lower than the lifetime of the donor, ensuring the availability of the acceptor for energy transfer. If the lifetime of the acceptor exceeds the lifetime of the donor, the acceptor might still be in the excited state when the donor is excited again. Advanced cyan fluorescent proteins such as mTurquoise show an extended lifetime and thus contribute to an increased probability of FRET (Figure1B). The probability of FRET also depends on the molar extinction coefficient of the acceptor.
Protokół
NOTE: For the following protocol, transient transfection of protoplasts was performed, as described previously12. A brief description is given below.
1. Transient transfection of protoplasts
- Cut ~4 g of healthy leaves of Arabidopsis thaliana ecotype Columbia into 1 mm slices and transfer them to 20 mL of enzyme solution (1.5% cellulase; 0.4% macerozyme; 0.1% bovine serum albumin Fraction V; 0.4 M mannitol; 20 mM KCl; 20 mM 2-(N-morpholino)ethanesulfonic acid (MES), pH 5.7; 10 mM CaCl2).
- Vacuum-infiltrate the leaf slices followed by an incubation with agitation for 2 h at room temperature. Harvest the cells by centrifugation for 3 min at 100 × g.
- Wash the protoplasts with W5-solution (154 mM NaCl; 125 mM CaCl2; 5 mM KCl; 2 mM MES, pH 5.7) and resuspend them in MMG solution (0.4 M mannitol; 15 mM MgCl2; 4 mM MES, pH 5.7).
- Perform the transfection in an 8-well slide by osmotic shock in the presence of polyethyleneglycol (PEG) 4000. Mix 20 µL of the protoplast suspension with 5 µL of plasmid DNA (5 µg/µL) and 25 µL of PEG solution (0.2 M mannitol, 0.1 M CaCl2, 40% PEG 4000).
- Reverse the osmotic shock by gentle readjustment of the osmotic conditions.
NOTE: Besides the sample of interest, the expression of donor alone and acceptor alone is required to determine the spectral bleed-through of the donor and the acceptor, respectively. A fusion protein of the donor and the acceptor must be expressed too for calibration purposes. The fluorescent protein expression was under the control of a cauliflower mosaic virus 35S promoter (pCaMV35S). For all measurements, two confocal laser scanning microscopes (LSM1 and LSM2) were used. LSM1 has two types of detectors: for FRET measurements, the donor signal was detected by a GaAsP-detector, while FRET and acceptor emission were recorded with a photomultiplier. LSM2 has two photomultipliers, which were used for the detection of donor, FRET, and acceptor emission.
2. Laser-adjustment
NOTE: Here, 458 nm and 514 nm lines of an argon-ion laser have been applied for FRET analysis between enhanced cyan fluorescent protein (ECFP)- and enhanced yellow fluorescent protein (EYFP)-labeled proteins. For reproducible data acquisition, both lines were adjusted to similar intensity. This was achieved by either a transmission photomultiplier or the reflection mode.
- Laser adjustment with a transmission photomultiplier
- Use an empty well for adjustment.
- Choose line-scanning mode and histogram view.
- Decrease the laser intensity to the minimum, and adjust the detector gain to detectable background noise.
- Increase the laser intensity in steps of 0.5% and record the corresponding signal.
- Apply the routine for both laser lines.
- Laser adjustment with reflection mode
- Use an empty well for adjustment.
- Apply a reflection filter, switch on the reflection mode, if available.
- Ensure that the detector wavelength range covers the wavelength of the laser.
- Choose the line-scanning mode and histogram view.
- Decrease the laser intensity to the minimum, and adjust the detector gain to detectable background noise.
- Move the objective to the lowest position.
- Move the objective up until the reflection of the coverslip is visible.
- Increase the laser intensity in steps of 0.5% and record the corresponding signal.
- Apply the routine for both laser lines.
- Data evaluation
- Tabulate the data and sort the data by signal intensities.
- Plot the signal intensities against the relative laser power.
- Choose laser intensities that result in similar signal intensity.
3. Adjustment of photomultipliers
NOTE: After laser adjustment, the photomultipliers were adjusted to individual gains to obtain similar sensitivity. This calibration was done with the 514 nm laser line, which is in the center of the wavelength range of interest.
- Use an empty well for adjustment.
- Apply a reflection filter, and switch to reflection mode if available.
- Ensure that the detector wavelength range covers the wavelength of the laser (514 nm).
- Choose the line scanning mode and histogram view.
- Decrease detector gain to half the maximum, and adjust the laser intensity to detectable background noise.
- Move the objective to the lowest position.
- Move the objective up until the reflection of the coverslip is visible.
- Increase the detector gain in steps of 50 to 100 V and record the corresponding signal.
- Apply steps 3.1 to 3.8 for both detectors.
- Data evaluation
- Plot the intensity against the detector gain for each detector.
- Choose the individual detector gains to obtain similar sensitivity.
4. FRET image acquisition
NOTE: Start with the sample of interest for setting up image acquisition.
- Choose the appropriate filters/dichroic mirrors, e.g., a double dichroic mirror MBS 458/514 for the FRET-pair ECFP/EYFP. Use the same dichroic mirror for all channels to enable line-by-line scanning. Select a water immersion objective for the imaging of living cells. Choose 12 bit- or 16-bit scanning and moderate scanning speed.
- Define the detection range, preferably 470-510 nm for donor detection and 530-600 nm for acceptor/FRET detection in the case of ECFP/EYFP. When using a 445 nm or 440 nm diode laser, use 450 to 510 nm as the detection range. In the case of an acousto-optic beam splitter (AOBS), define donor detection in the range of 450 to 500 nm to prevent unwanted acceptor detection.
- Apply the detector setting according to 3.10.2.
- Apply the laser setting according to 2.3.2. Revise the laser intensity based on the obtained laser power table, if required. Ensure that the signal-to-noise ratio covers the entire dynamic range of the detectors (intensity ranging from 0 to 4095 for 12-bit scanning).
- Keep laser intensities and detector gains constant. Use the pinhole diameter for fine-tuning.
NOTE: Keep in mind that changes in the pinhole diameter affect spatial resolution. - Perform the measurements (take images of at least 20 cells).
5. Determination of crosstalk corrections
NOTE: Cells expressing only the donor or the acceptor are required to determine donor spectral bleed-through (DSBT) and acceptor spectral bleed-through (ASBT), respectively. Keep the same settings described in section 4.
- Perform FRET measurements with cells expressing the donor fluorophore.
- Perform FRET measurements with cells expressing the acceptor fluorophore.
6. Calibration of the measurements according to Beemiller et al.13
NOTE: Cells expressing a donor-acceptor fusion of known FRET efficiency are required. Here, an ECFP-5 aa-EYFP-fusion with a FRET efficiency of 0.46 has been used4. Keep the same settings described in section 4.
- Perform FRET measurements with cells expressing the donor-acceptor fusion
7. Data evaluation
- Obtain line profiles of the cells, ensuring that each profile contains no more than one cell. Save the profiles as text files.
- Import the text files into a spreadsheet using the text file import option in the Data section.
- Read out the maximum values by applying the Max function.
- List the obtained values in a table, have a column each for donor emission ID, FRET emission IF, acceptor emission IA, and at least four data sets: donor only, acceptor only, donor-acceptor fusion, and measurement.
NOTE: Excitation of the donor also results in direct excitation of the acceptor and causes ASBT that is described by the α value. - Calculate the ASBT α values with the acceptor-only dataset using equation (1).
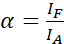 (1)
(1)
NOTE: Use the median of all α-values in the following equations. The donor shows a broad emission spectrum that results in emission crosstalk with the sensitized emission of the acceptor. This DSBT is given by the β value. - Calculate the donor spectral bleed-through β values with the donor-only dataset using equation (2).
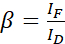 (2)
(2)
NOTE: Use the median of all β values in the following equations. The calibration factor ξ describes the linear relationship of FRET-derived donor quenching and sensitized emission of the acceptor. Use the medians of 7.5 and 7.6 in the following equations. - Calculate the calibration factors ξ with the donor-acceptor fusion dataset and its FRET efficiency E (0.46) using equation (3).
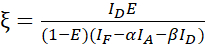 (3)
(3)
NOTE: Use the median of all ξ values in the following equations. - Calculate the FRET efficiencies of the protein pair of interest using equations (4) and (5).
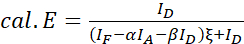 (4)
(4)
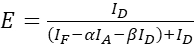 (5)
(5) - Estimate the effects of expression strength and/or donor-acceptor ratio: plot the sum of ID, IF, and IA against the FRET efficiencies. Perform a linear regression; note that the steeper the graph and the higher R2 is, the higher is the impact of the expression level or the greater is the difference of donor and acceptor abundance.
Wyniki
Adjustment of the confocal laser-scanning microscope
The laser adjustment revealed a linear increase of emission with increasing laser intensity (Figure 2 and Table 1). As expected for argon-ion lasers, the emission of the 514 nm line was much higher than the emission of the 458 nm line, as evidenced by a steeper slope. For subsequent experiments, laser power of 4.5% and 6.5% was chosen for the 514 nm line and the 458 nm line, respectively. This result...
Dyskusje
Donor quenching and sensitized acceptor emission are characterized by a linear relationship that allows for either donor- or acceptor-based calculation of FRET. The corresponding factors of linearity are called either G factor (donor to acceptor) or xi (acceptor to donor), which are reciprocal values4. Measuring FRET between fluorescent proteins by fluorescence microscopy often requires corrections for DSBT and ASBT due to the broad absorption and emission spectra of the fluorescent proteins. Howe...
Ujawnienia
We ensure that all authors have disclosed any and all conflicts of interest and have no competing financial interests.
Podziękowania
The experiments were performed at the Light Microscopy Technology Platform (LiMiTec) of the Faculty of Biology, Bielefeld University. This work has been funded by Bielefeld University.
Materiały
| Name | Company | Catalog Number | Comments |
| 8-well slides | Ibidi | 80821 | |
| Immersion oil Immersol W2010 | Zeiss | 444969-0000-000 | refraction index of water |
| LSM 1: AxioObserver with LSM 780 scan head, confocal laser scanning microscope | Zeiss | ||
| LSM 2: AxioObserver with LSM 5 scan head, confocal laser scanning microscope | Zeiss |
Odniesienia
- Lakowicz, J. R. . Principles of Fluorescent Spectroscopy. Third Edition. , (2006).
- Clegg, R. M. Förster resonance energy transfer- FRET what it is, why do it, and how it's done. Laboratory Techniques in Biochemistry and Molecular Biology. 33, 1-57 (2009).
- Vogel, S. S., Nguyen, T. A., vander Meer, B. W., Blank, P. S. The impact of heterogeneity and dark acceptor states on FRET: implications for using fluorescent protein donors and acceptors. PLoS ONE. 7, 49593 (2012).
- Müller, S. M., Galliardt, H., Schneider, J., Barisas, B. G., Seidel, T. Quantification of Förster resonance energy transfer by monitoring sensitized emission in living plant cells. Frontiers in Plant Science. 4, 413 (2013).
- Gadella, T. W. J., vander Krogt, G. N., Bisseling, T. GFP-based FRET-microscopy in living plant cells. Trends in Plant Science. 4, 287-291 (1999).
- Van Rheenen, J., Langeslag, M., Jalink, K. Correcting confocal acquisition to optimize imaging of fluorescence resonance energy transfer by sensitized emission. Biophysical Journal. 86, 2517-2529 (2004).
- Seidel, T., Golldack, D., Dietz, K. J. Mapping of C-termini of V-ATPase subunits by in vivo-FRET measurements. FEBS Letters. 579, 4374-4382 (2005).
- Seidel, T., Schnitzer, D., Golldack, D., Sauer, M., Dietz, K. J. Organelle-specific iso-enzymes of plant V-ATPase as revealed by in vivo-FRET. BMC Cell Biology. 9, 28 (2008).
- Schnitzer, D., Seidel, T., Sander, T., Golldack, D., Dietz, K. J. The cellular energization state affects peripheral stalk stability of plant vacuolar H+-ATPase and impairs vacuolar acidification. Plant Cell Physiology. 52, 946-956 (2011).
- Roshchina, V. V. Vital autofluorescence: application to the study of plant living cells. International Journal of Spectroscopy. 2012, 124672 (2012).
- Holtorf, S., Apel, K., Bohlmann, H. Comparison of different constitutive and inducible promoters for the overexpression of transgenes in Arabidopsis thaliana. Plant Molecular Biology. 29, 637-646 (1995).
- Seidel, T., et al. Colocalization and FRET-analysis of subunits c and a of the vacuolar H+-ATPase in living plant cells. Journal of Biotechnology. 112 (1-2), 165-175 (2004).
- Beemiller, P., Hoppe, A. D., Swanson, J. A. A phosphatidylinositol-3-kinase-dependent signal transition regulates ARF1 and ARF6 during FCγ receptor-mediated phagocytosis. PLoS Biology. 4, 162 (2006).
- Lambert, T. J. FPbase: a community-editable fluorescent protein database. Nature Methods. 16, 277-278 (2019).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone