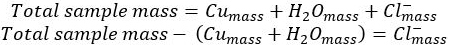Determining the Empirical Formula
Обзор
Source: Laboratory of Dr. Neal Abrams - SUNY College of Environmental Science and Forestry
Determining the chemical formula of a compound is at the heart of what chemists do in the laboratory every day. Many tools are available to aid in this determination, but one of the simplest (and most accurate) is the determination of the empirical formula. Why is this useful? Because of the law of conservation of mass, any reaction can be followed gravimetrically, or by change in mass. The empirical formula provides the smallest whole-number ratio among elements (or compounds) within a molecular compound. In this experiment, gravimetric analysis will be used to determine the empirical formula of copper chloride hydrate, CuxCly·nH2O.
Процедура
1. Dehydrating the Hydrate
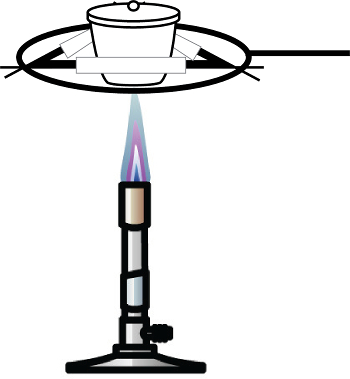
- Accurately weigh a sample of copper chloride hydrate and place it into a pre-dried and tared crucible. It is important that the crucible is dried above 120 °C to drive off any adsorbed moisture. Typically, 1–2 g of compound will suffice.
- Heat the sample using a Bunsen burner or other flame source until it changes color from greenish-blue to a reddish-brown (Figure 1). This color change is indicative of the anhydrous form of copper chloride.
Результаты
- Experiment
- Heat 1.25 g of copper chloride hydrate in a crucible. After heating and then cooling, the final mass is 0.986 g of copper chloride, CuxCly.
- Dissolve the CuxCly sample in 50 mL of deionized water and add 0.2 g of fine aluminum mesh to the beaker.
- After reacting and dissolving the excess aluminum, 0.198 g of dried copper metal is recovered.
- Subtract the mass of both copper and water from the initial copper chloride hydrate to yield&#
Заявка и Краткое содержание
In one example, suppose an unknown biomolecule containing only C, H, and O is found to act well as a new fuel. One way to determine the formula of the fuel would be to combust it in air and analyze the products:
CxHyOz + O2 → mCO2 + nH2O
While O2 is in excess, we would know all the carbon in CO2 originated from the biomolecule and all the hydrogen would be present in H
Перейти к...
Видео из этой коллекции:

Now Playing
Determining the Empirical Formula
General Chemistry
183.8K Просмотры

Common Lab Glassware and Uses
General Chemistry
659.1K Просмотры

Solutions and Concentrations
General Chemistry
275.4K Просмотры

Determining the Density of a Solid and Liquid
General Chemistry
557.0K Просмотры

Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
383.9K Просмотры

Determining the Solubility Rules of Ionic Compounds
General Chemistry
141.6K Просмотры

Using a pH Meter
General Chemistry
347.0K Просмотры

Introduction to Titration
General Chemistry
425.8K Просмотры

Ideal Gas Law
General Chemistry
79.4K Просмотры

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
158.9K Просмотры

Le Châtelier's Principle
General Chemistry
265.8K Просмотры

Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
160.8K Просмотры

Determining Rate Laws and the Order of Reaction
General Chemistry
196.4K Просмотры

Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.8K Просмотры

Coordination Chemistry Complexes
General Chemistry
91.8K Просмотры
Авторские права © 2025 MyJoVE Corporation. Все права защищены