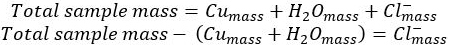Déterminer la formule empirique
Vue d'ensemble
Source : Laboratoire du Dr Neal Abrams - SUNY College de foresterie et sciences de l’environnement
Déterminer la formule chimique d’un composé est au cœur de ce font chimistes en laboratoire tous les jours. De nombreux outils sont disponibles pour aider à cette décision, mais un des plus simple (et plus précis) est la détermination de la formule empirique. Pourquoi est-ce utile ? En raison de la Loi de conservation de la masse, toute réaction peut être suivie par gravimétrie, ou par changement de masse. La formule empirique fournit le plus petit nombre entier ratio entre les éléments (ou composés) au sein d’un composé moléculaire. Dans cette expérience, analyse gravimétrique serviront à déterminer la formule empirique de l’hydrate de chlorure de cuivre, CuxCly·nH2O.
Procédure
1. la mise en attente de l’Hydrate
- Peser un échantillon de l’hydrate de chlorure de cuivre et placez-le dans un creuset préalablement séché et taré. Il est important que le creuset est séché au-dessus de 120 ° C pour chasser toute trace d’humidité adsorbée. En règle générale, 1 à 2 g de composé suffira.
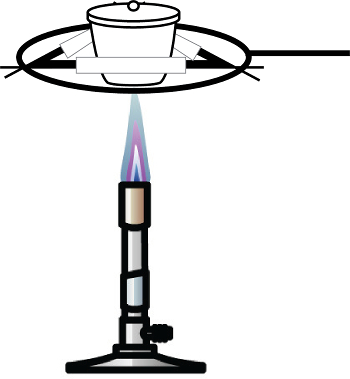
- Chauffer l’échantillon à l’aide d’un bec Bunsen ou une autre source de flamme jusqu'à ce qu’elle change de couleur de bleu verdâtre à un rouge-brun (Figure 1). Ce changement de coule
Résultats
- Experiment
- 1,25 g d’hydrate de chlorure de cuivre dans un creuset de la chaleur. Après chauffage et refroidissement puis, la messe finale est 0,986 g de chlorure de cuivre, CuxCly.
- Dissoudre l’échantillon CuxCly dans 50 mL d’eau désionisée et ajouter 0,2 g d’aluminium fine maille dans le bécher.
- Après réagissant et en dissolvant l’aluminium excédentaire, 0,198 g sec du métal de cuivre est récupéré.
- Soustraire de la masse de cui...
Applications et Résumé
Par exemple, supposons une biomolécule inconnue contenant seulement C, H, et O se trouve à agir ainsi qu’un nouveau combustible. Une façon de déterminer la formule du carburant aurait soit-il pour la combustion dans l’air et d’analyser les produits :
CxHyOz + O2 → AGC2 nH2O
O2 est en excès, nous saurions tout le carbone en CO2 provient de la biomolécule et tous l?...
Passer à...
Vidéos de cette collection:

Now Playing
Déterminer la formule empirique
General Chemistry
182.8K Vues

Présentation et utilisation de la verrerie de laboratoire courante
General Chemistry
657.3K Vues

Solutions et Concentrations
General Chemistry
274.6K Vues

Déterminer la densité d'un solide et d'un liquide
General Chemistry
556.5K Vues

Déterminer la concentration massique d'une solution aqueuse
General Chemistry
383.7K Vues

Détermination des règles de solubilité des composés ioniques
General Chemistry
141.5K Vues

Utiliser un pH-mètre
General Chemistry
346.5K Vues

Introduction au titrage
General Chemistry
425.1K Vues

Loi des gaz parfaits
General Chemistry
78.6K Vues

Détermination spectrophotométrique d'une constante d'équilibre
General Chemistry
158.6K Vues

Principe de Le Châtelier
General Chemistry
265.7K Vues

Identification d'un composé inconnu grâce à la loi de la cryométrie
General Chemistry
160.7K Vues

Détermination de la loi de vitesse et de l'ordre de la réaction
General Chemistry
196.2K Vues

Utilisation de la calorimétrie différentielle à balayage pour mesurer les changements d'enthalpie
General Chemistry
44.5K Vues

Complexes de coordination
General Chemistry
91.6K Vues