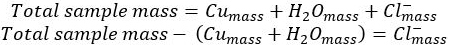Determinação da Fórmula Empírica
Visão Geral
Fonte: Laboratório do Dr. Neal Abrams - SUNY College of Environmental Science and Forestry
Determinar a fórmula química de um composto está no centro do que os químicos fazem no laboratório todos os dias. Muitas ferramentas estão disponíveis para auxiliar nessa determinação, mas uma das mais simples (e mais precisas) é a determinação da fórmula empírica. Por que isso é útil? Por causa da lei de conservação da massa, qualquer reação pode ser seguida gravimetricamente, ou por mudança de massa. A fórmula empírica fornece a menor razão de número inteiro entre elementos (ou compostos) dentro de um composto molecular. Neste experimento, a análise gravimétrica será utilizada para determinar a fórmula empírica do hidrato de cloreto de cobre,xCly·nH2O.
Procedimento
1. Desidratando o Hidratado
- Pesar com precisão uma amostra de hidrato de cloreto de cobre e colocá-lo em um cadinho pré-seco e tared. É importante que o cadinho esteja seco acima de 120 °C para afastar qualquer umidade adsorvida. Normalmente, 1-2 g de composto será suficiente.
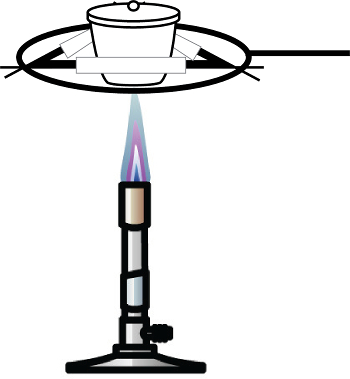
- Aqueça a amostra usando um queimador Bunsen ou outra fonte de chama até que ela mude de cor de azul esverdeado para marrom-avermelhado(Figura 1). Esta mudança de cor é indicativa da forma anidro de cloreto de co
Resultados
- Experimentar
- Aqueça 1,25 g de hidrato de cloreto de cobre em um cadinho. Após o aquecimento e depois o resfriamento, a massa final é de 0,986 g de cloreto de cobre,xCly.
- Dissolva a amostraxCly em 50 mL de água deionizada e adicione 0,2 g de malha de alumínio fino ao béquer.
- Após reagir e dissolver o excesso de alumínio, recupera-se 0,198 g de metal de cobre seco.
- Subtraia a massa de cobre e água do hidrato inicial de cloreto de cobre para produ...
Aplicação e Resumo
Em um exemplo, suponha que uma biomolécula desconhecida contendo apenas C, H e O é encontrada para agir bem como um novo combustível. Uma maneira de determinar a fórmula do combustível seria combustá-lo no ar e analisar os produtos:
CxHyOz + O2 → mCO2 + nH2O
Enquanto O2 está em excesso, saberíamos que todo o carbono em CO2 originou-se da biomolécula e todo o hidrogêni...
Pular para...
Vídeos desta coleção:

Now Playing
Determinação da Fórmula Empírica
General Chemistry
180.4K Visualizações

Vidraria de laboratório comuns e seus usos
General Chemistry
654.2K Visualizações

Soluções e Concentrações
General Chemistry
273.2K Visualizações

Determinando a densidade de um sólido e um líquido
General Chemistry
555.0K Visualizações

Determinação de composição percentual em massa em uma solução aquosa
General Chemistry
383.1K Visualizações

Determinação das Regras de Solubilidade de Compostos Iônicos
General Chemistry
141.1K Visualizações

Usando um medidor de pH
General Chemistry
344.5K Visualizações

Introdução à Titulação
General Chemistry
423.5K Visualizações

Lei dos gases ideais
General Chemistry
78.2K Visualizações

Determinação espectrofotométrica de uma constante de equilíbrio
General Chemistry
158.3K Visualizações

Princípio de Le Châtelier
General Chemistry
264.2K Visualizações

Depressão do ponto de congelamento para determinar um composto desconhecido
General Chemistry
160.5K Visualizações

Determinação das Leis de Velocidade e da Ordem de Reação
General Chemistry
195.7K Visualizações

Uso de Calorimetria de Varredura Diferencial para Medir Mudanças na Entalpia
General Chemistry
44.4K Visualizações

Complexos de Química de Coordenação
General Chemistry
91.2K Visualizações
Copyright © 2025 MyJoVE Corporation. Todos os direitos reservados