20.18 : Maxwell's Thermodynamic Relations
Maxwell's thermodynamic relations are very useful in solving problems in thermodynamics. Each of Maxwell's relations relates a partial differential between quantities that can be hard to measure experimentally to a partial differential between quantities that can be easily measured. These relations are a set of equations derivable from the symmetry of the second derivatives and the thermodynamic potentials.
All thermodynamic potentials are exact differentials. Therefore, their second-order derivative does not depend on the order of differentiation. In the case of Maxwell's relations, the thermodynamic potential is expressed in terms of the partial derivatives of that function. Substituting the values of the partial derivatives gives Maxwell's equations. The four Maxwell's relations are:
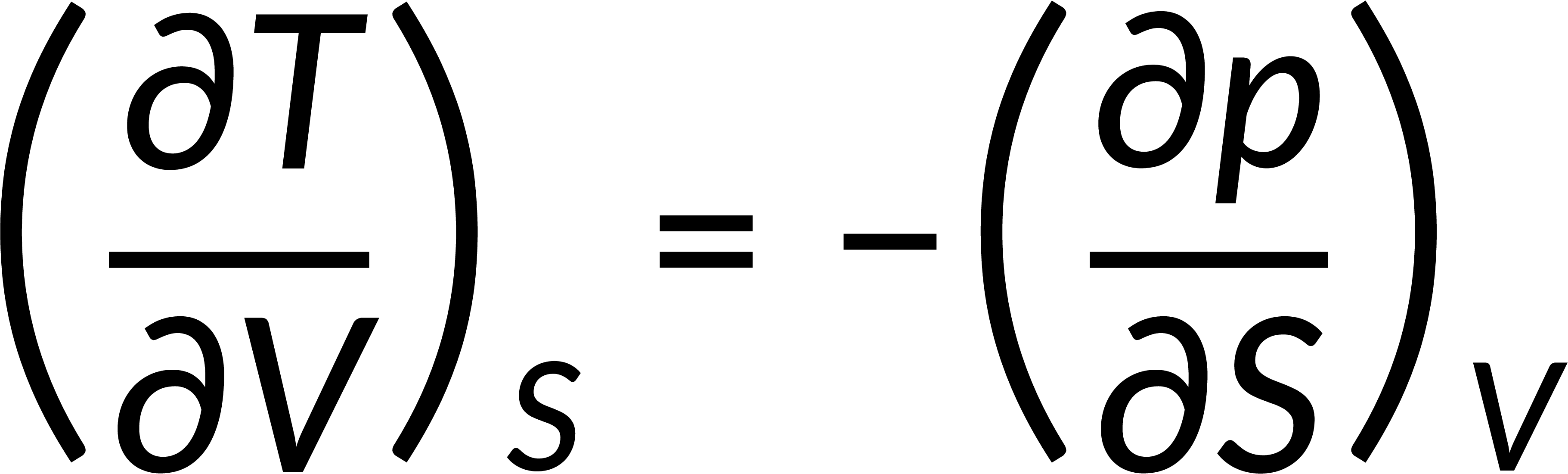
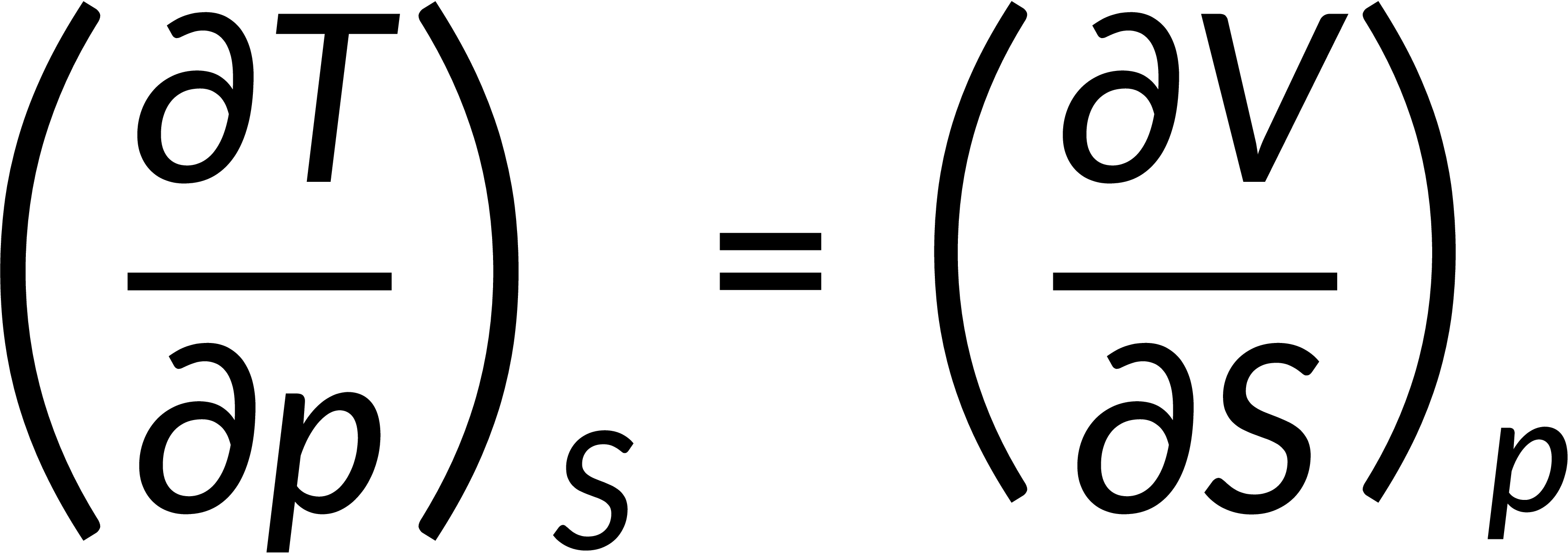
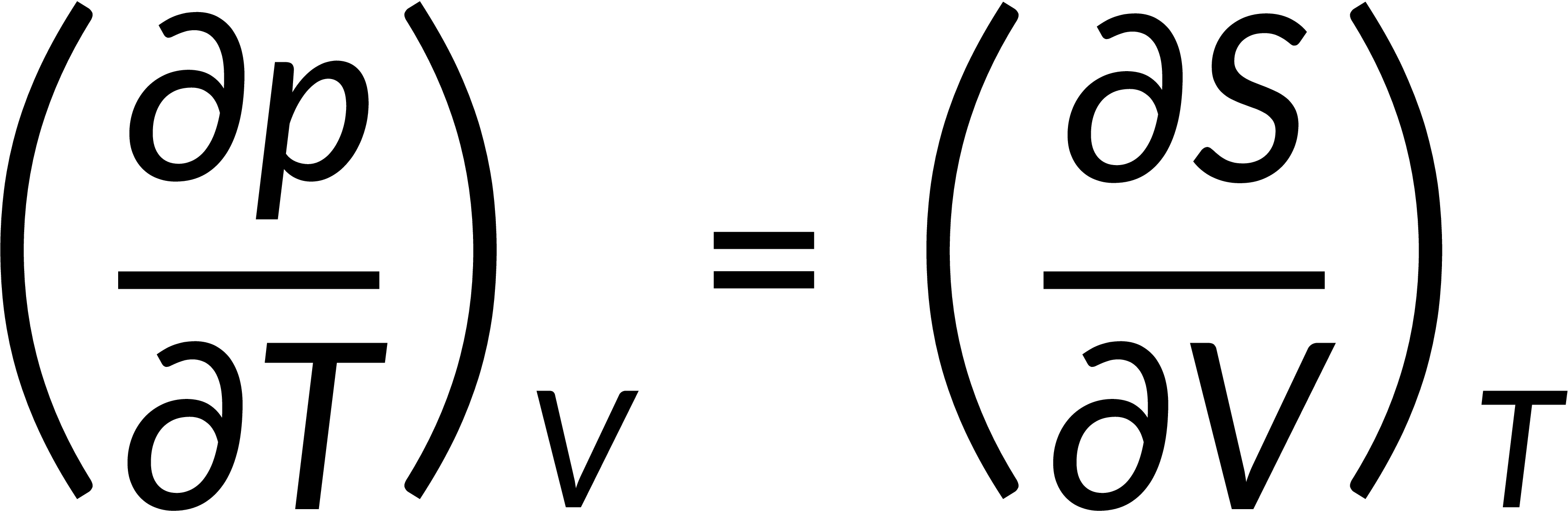
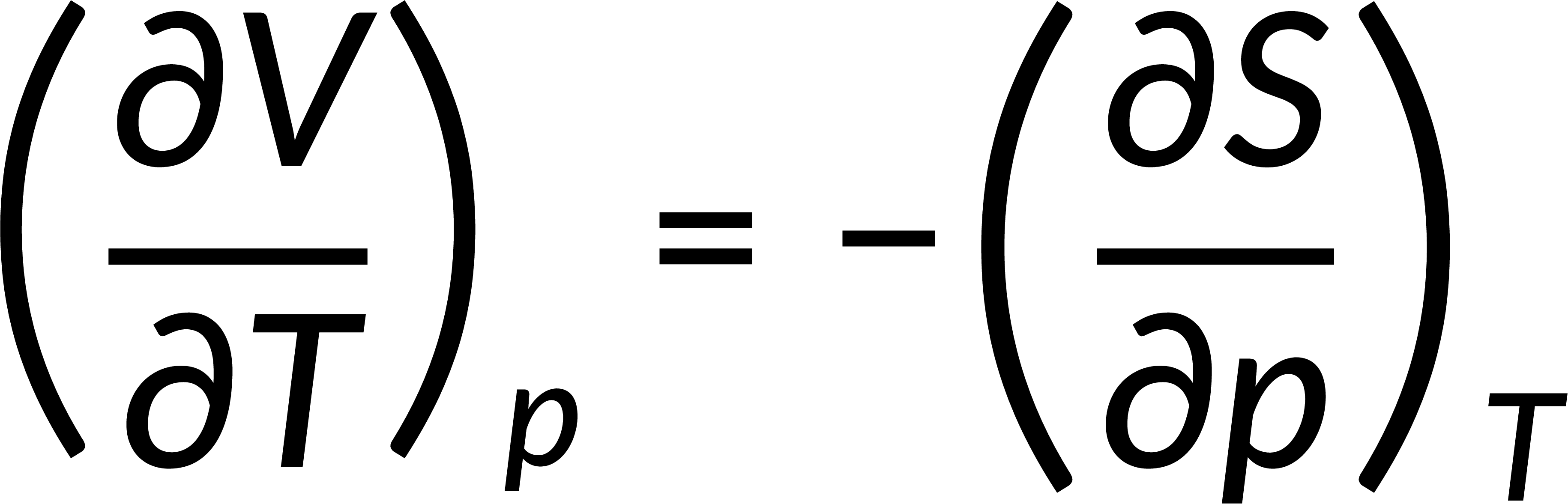
These equations relate entropy changes, which are difficult to measure, with changes in other thermodynamic variables, like temperature, volume, and pressure, that are easier to measure. For example, in the last equation, the left-hand side term gives the change in pressure with the temperature at constant volume. This quantity can be easily measured in a laboratory. However, the term on the right-hand side of the equation is more complicated, as it is hard to measure the entropy change with volume at a constant temperature.
From Chapter 20:

Now Playing
20.18 : Maxwell's Thermodynamic Relations
The First Law of Thermodynamics
2.6K Views

20.1 : Thermodynamic Systems
The First Law of Thermodynamics
5.0K Views

20.2 : Work Done During Volume Change
The First Law of Thermodynamics
3.9K Views

20.3 : Path Between Thermodynamics States
The First Law of Thermodynamics
3.1K Views

20.4 : Heat and Free Expansion
The First Law of Thermodynamics
1.8K Views

20.5 : Internal Energy
The First Law of Thermodynamics
4.5K Views

20.6 : First Law of Thermodynamics
The First Law of Thermodynamics
4.1K Views

20.7 : First Law Of Thermodynamics: Problem-Solving
The First Law of Thermodynamics
2.5K Views

20.8 : Cyclic Processes And Isolated Systems
The First Law of Thermodynamics
2.7K Views

20.9 : Isothermal Processes
The First Law of Thermodynamics
3.5K Views

20.10 : Isochoric and Isobaric Processes
The First Law of Thermodynamics
3.4K Views

20.11 : Heat Capacities of an Ideal Gas I
The First Law of Thermodynamics
2.6K Views

20.12 : Heat Capacities of an Ideal Gas II
The First Law of Thermodynamics
2.4K Views

20.13 : Heat Capacities of an Ideal Gas III
The First Law of Thermodynamics
2.2K Views

20.14 : Adiabatic Processes for an Ideal Gas
The First Law of Thermodynamics
3.0K Views
See More
Copyright © 2025 MyJoVE Corporation. All rights reserved