Method Article
Antimicrobial Susceptibility Testing of Mycobacterium Tuberculosis Complex for First and Second Line Drugs by Broth Dilution in a Microtiter Plate Format
In This Article
Summary
Antimicrobial susceptibility testing of Mycobacterium tuberculosis is challenging but critical for patient care. This microtiter plate format offers testing of 12 antimycobacterial drugs and provides minimum inhibitory concentration (MIC) values, which will aid in appropriate treatment.
Abstract
The rapid detection of antimicrobial resistance is important in the effort to control the increase in resistant Mycobacterium tuberculosis (Mtb). Antimicrobial susceptibility testing (AST) of Mtb has traditionally been performed by the agar method of proportion or by macrobroth testing on an instrument such as the BACTEC (Becton Dickinson, Sparks, MD), VersaTREK (TREK Diagnostics, Cleveland, OH) or BacT/ALERT (bioMérieux, Hazelwood, MO). The agar proportion method, while considered the “gold” standard of AST, is labor intensive and requires calculation of resistance by performing colony counts on drug-containing agar as compared to drug-free agar. If there is ≥1% growth on the drug-containing medium as compared to drug-free medium, the organism is considered resistant to that drug. The macrobroth methods require instrumentation and test break point ("critical") drug concentrations for the first line drugs (isoniazid, ethambutol, rifampin, and pyrazinamide). The method described here is commercially available in a 96 well microtiter plate format [MYCOTB (TREK Diagnostics)] and contains increasing concentrations of 12 antimicrobials used for treatment of tuberculosis including both first (isoniazid, rifampin, ethambutol) and second line drugs (amikacin, cycloserine, ethionamide, kanamycin, moxifloxacin, ofloxacin, para-aminosalicylic acid, rifabutin, and streptomycin). Pyrazinamide, a first line drug, is not included in the microtiter plate due to its need for acidic test conditions. Advantages of the microtiter system include both ease of set up and faster turn around time (14 days) compared with traditional agar proportion (21 days). In addition, the plate can be set up from inoculum prepared using either broth or solid medium. Since the microtiter plate format is new and since Mtb presents unique safety challenges in the laboratory, this protocol will describe how to safely setup, incubate and read the microtiter plate.
Protocol
Each laboratory needs to perform a risk assessment in collaboration with their Institutional Biological Safety Officer to determine the appropriate biosafety level for preparation of the inoculum and for pipetting of the inoculum into the plate. In our laboratory, we utilize biosafety level 3 laboratory (BSL3) practices until the microtiter plates are inoculated, and sealed with a plastic adhesive seal. These plates are then placed inside a plastic bag, which is heat-also sealed. Incubation and interpretation reading of the microtiter plate is conducted in a biosafety level 2 laboratory (BSL2).
1. Labeling of materials
- Label a blood agar plate, three Middlebrook 7H10 plates, Middlebrook 7H9 broth, a saline tween glass bead tube and a microtiter AST plate with the appropriate identifiers (e.g., patient name, medical record number) in the BSL2.
- Label the blood agar and 7H10 plated as “purity check”. Periodic colony counts of the inoculum are recommended to ensure that an appropriate concentration of organisms is used in the test. Colony counts when performed will need to have two of the 7H10 plates labeled with “1/1 colony count” and “1/50 colony count”.
2. Technologist preparations to go into the BSL3
- Appropriate personal protective equipment is necessary to work in the BSL3 and includes solid-front gown, head cover, gloves, shoe covers, and a respirator. Each institution should establish their own respiratory protection plan in collaboration with their institution’s Industrial Hygiene Officer. In our laboratory, we have several respirators available, including powered air purifying respirators (PAPRs) and fitted HEPA-filtered, N95 half-face respirators.
3. Preparation of the biological safety cabinet
- In the BSL3, prepare the biological safety cabinet (BSC) by disinfecting the surface and placing a paper towel soaked with disinfectant approximately 6 inches from the air vent panel.
- Place a waste container on the left, and small nephlometer and vortexer on the right.
- Inoculating loops, pipetors and tips are placed beside the paper towel.
- Place a rack with the organism (in broth or on solid medium) and the McFarland 0.5 standard on the paper towel.
4. Calibration of the nephelometer
- Place a saline tween glass bead tube in the nephelometer and set the instrument to zero using the saline tween blank.
- Place the McFarland 0.5 standard in the nephelometer and calibrate according the manufacturer's instructions.
5. Preparation of the Inoculum
- Working in the BSC, scrape colonies from solid medium using a sterile loop into the appropriately labeled saline tween glass bead tube until a ≥0.5 McFarland Standard is obtained.
- Visually inspect the sample, and compare to the McFarland Standard.
- Vortex sample for 30-60 seconds.
- Allow the inoculum to settle for 15 minutes (set a timer).
6. Inoculation of the microtiter plate
- Adjust the inoculum with saline tween broth using the nephelometer calibrated to the McFarland 0.5 in steps 4.1 and 4.2. This must be completed within 30 minutes (per manufacturer specifications).
- Use a 200 μL pipetter and a LONG aerosol resistant pipet tip to remove 100 μL of inoculum from the TOP 1/3rd of the saline bead tween tube. Inoculate the Middlebrook 7H9 Broth. Slowly vortex the inoculated 7H9 broth for 20 seconds.
- Remove the AST microtiter plate from manufacturer's package. Do NOT use if the desiccant is blue or if the foil package is damaged.
- Prepare a 1:50 dilution tube for use later in the preparation of the 1:50 dilution by pipetting 50 μL of sterile water into a sterile tube.
- Carefully pour the 7H9 broth inoculum into a trough. AVOID GENERATING AEROSOLS.
- Place the AST microtiter plate with the label facing you.
- Use a 12-channel pipetter to add 100 μL of the inoculum to each well.
- Prepare dilutions for colony counts:
- The 1/1 dilution is prepared from the positive control well (H12) by streaking 1 μl loop on the 7H10 plate labeled “1/1”.
- The 1/50 dilution is prepared by inoculating a 1 μl loop from the positive control well (H12) and swirling it into the tube with 50 μl of water tube prepared above. Streak 1 μL of this dilution to the plate labeled “1/50”.
- Inoculate the purity plates from the trough by pipetting 50 μL onto an appropriately labeled blood agar and 7H10 plate and streak for isolation.
- Place an adhesive seal on the AST microtiter plate. Press firmly on each well to assure adequate sealing by using the white back of the seal. Avoid creases.
- Disinfect the microtiter plate by wiping it with a paper towel soaked with a tuberculocidal disinfectant and place microtiter plate in a plastic bag and close with a heat seal or tape.
- The plastic bag serves as a secondary container to ensure that all broth is contained should there be a leak or spill of the primary plate.
7. Disinfection of materials and biological safety cabinet
- To avoid producing aerosols, place another trough on top of the inoculated trough before placing it gently into the discard container.
- Disinfect all materials including nephlometer, vortexer, plates, tubes, etc. by wiping down with a tuberculocidal disinfectant and wait the appropriate time to insure disinfection according to the manufacturer’s instructions before removing from the BSC. For example, Prospray, the tuberculocidal disinfectant we use, requires 15 minutes to kill mycobacteria.
- Place the discard container in a biohazard bag and seal before autoclaving.
8. Incubation
- Plates should be incubated with media down and care should be taken to avoid spilling or splashing any liquid.
- Incubate the AST microtiter plate plate, 7H10 and blood agar plates in a 35-37°C incubator with 5-10% CO2 with 5-10% CO2 in the BSL2 laboratory.
- In our laboratory, this is acceptable because the plate is well-sealed and inside a sealed plastic bag.
- No more than 2 microtiter AST plates should be stacked in the incubator according to the manufacturer’s instructions.
9. Representative results
Interpretation of results:
Sealed AST microtiter plates can be read manually using a mirror as an aid or with an automated plate reader such as the Vizion (TREK Diagnostics). The plates can be examined as soon as 7 days after inoculation. Examine the growth control wells in positions H11 and H12 to determine if they are positive (ie., have a deposit of cells at the bottom of the well). If the growth control wells are not positive, reincubate the plate and re-examine periodically (eg., day 10, day 14) until the growth control wells are positive.
- Mirror read:
- Place sealed AST microtiter plate onto the platform;
- Read growth control wells after 7 days of incubation; if this is negative, reincubate AST microtiter plate;
- If the growth controls are acceptable, then read the drug-containing wells. For for each drug, record the first dilution that has no growth by comparing the drug wells with the growth control.
- Growth appears as turbidity or as a deposit of cells at the bottom of a well.
- Instrument Read: follow the manufacturers instructions; the instructions below are an example of how the reading would be done with the Vizion platform:
- Log in.
- Use “MYCOTB” AST plate software.
- Place sealed plate with the barcode facing the user. Growth appears as turbidity or as a deposit of cells at the bottom of a well.
- Read growth controls; if this is negative, reincubate AST microtiter plate; if positive read the plate by touching the screen with the first well of each drug that does not show growth.
Read purity plates. The blood agar plate should show no growth after 48 hours and the 7H10 purity plate should have confluent growth of one organism type. If contaminated, the AST microtiter test needs to be repeated from a pure culture.
Calculate colony counts from the positive control well using the table below:
Colony Count Key
| # colonies counted on plate labeled "1/1" | # colonies counted on plate labeled "1/50" | Inoculum Colony Count (cfu/mL) |
| <50 | 0 | <5 X 104 |
| 50-100 | 0-2 | 5 X 104 to 1 X 105 |
| >100 | ≤ 10 | 1 X 105 to 5 X 105 |
| >100 | >10 | >5 X 105 |
There may be trailing with para-aminosalicylic acid in some strains of Mycobacterium tuberculosis. This can be interpreted by looking at all wells that have pellets and using the first well that has the same size pellet as the next dilution as the end point. Resistance is rare with this drug.
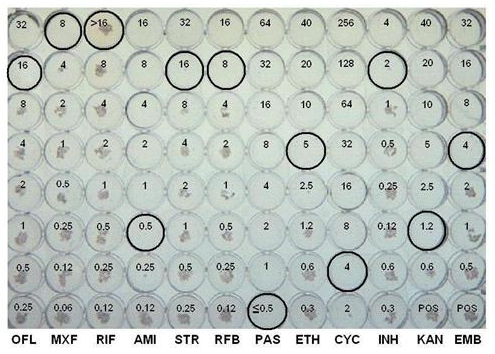
Figure 1. Representative results using the MYCOTB plate.
| Drug abbreviation | Drug | MIC μ/mL |
| OFL | ofloxacin | 16 |
| MXF | moxifloxacin | 8 |
| RIF | rifampin | >16 |
| AMI | amikacin | 0.5 |
| STR | streptomycin | 16 |
| RFB | rifabutin | 8 |
| PAS | p-aminosalicylic acid | ≤0.5 |
| ETH | ethionamide | 5 |
| CYC | cycloserine | 4 |
| INH | isoniazid | 2 |
| KAN | kanamycin | 1.2 |
| EMB | ethambutol | 4.0 |
Discussion
An example of a AST microtiter plate that has positive growth controls is shown in Figure 1 and the endpoint MIC (μg/ml) for each drug is circled. A study conducted in our laboratory indicated that the AST microtiter plate method is equivalent to the gold standard agar proportion method for susceptibility testing of Mycobacterium tuberculosis. Faster reporting of results was obtained with microtiter AST microtiter plate when compared to the traditional agar proportion method with the final interpretation for AST microtiter plate and agar proportion method at 14 and 21 days, respectively.The AST microtiter plate has the advantage of testing first and second line drugs simultaneously which can help prevent inordinate delays with resistant strains. The AST microtiter plate is also much easier to set up and read than the APM and both a manual mirror and a semi-automated system such as the VIZION can be used to read the plates. The availability of a MIC value for TB drug resistance testing may provide valuable new information to physicians relative to the traditional critical concentration value, especially for those isolates with “borderline” susceptibility.
Disclosures
There is nothing to disclose.
Acknowledgements
We would like to thank the laboratory technologists of the Mayo Clinic Mycobacteriology Laboratory for their help during the study.
Materials
| Name | Company | Catalog Number | Comments |
| AST microtiter plates and adhesive seal | TREK Diagnostics, Thermo Scientific | MYCOTB | |
| McFarland 0.5 standard | TREK Diagnostics, Thermo Scientific | E1041 | |
| Middlebrook 7H10 plates | Fisher Scientific | ||
| Blood Agar Plates | Fisher Scientific | ||
| Sterile loops 1 μL | Fisher Scientific | ||
| Sterile loops 10 μL | Fisher Scientific | ||
| Sensititre Saline-Tween glass bead broth | TREK Diagnostics, Thermo Scientific | 27143 | |
| 7H9 OADC Broth | TREK Diagnostics, Thermo Scientific | T3440 | |
| Long Pipet Tips | Fisher Scientific | ||
| Sterile Troughs | TREK Diagnostics, Thermo Scientific | ||
| M. tuberculosis | ATCC | 27294 | |
| Pipeters: 1000, 200, 20μL | Fisher Scientific | ||
| Tips 1000, 20, 20 μL | Fisher Scientific | ||
| Multichannel Pipetter | Fisher Scientific | ||
| Incubator 5-10% CO2 | Fisher Scientific | ||
| Plastic bags, small, CO2 permeable | Poly Plastic Products | ||
| TB disinfectant (Such as ProSpray C-60) | Certol International, Commerce City, CO | PSC60/32 | |
| Mirror Box Reader | Fisher Scientific |
References
- Wengenack, N., Hall, L., Labombardi, V., Parrish, N. Evaluation of the New Sensititre (MYCOTB) MIC Plate for the Susceptibility Testing of Mycobacterium tuberculosis (Mtb) to First and Second Line Drugs. , (2010).
- . HHS Publication No. (CDC) 21-112: Biosafety in Microbiological and Biomedical Laboratories (BMBL). U.S. Department of Health and Human Services Centers for Disease and Preventions. , (2009).
- Morcillo, N., Imperiale, B., Digiulio, B. Evaluation of MGIT 960 and the colorimetric-based method for tuberculosis drug susceptibility testing. Int J Tuberc Lung Dis. 14, 1169-1175 (2010).
- Bemer, P., Bodmer, T., Munzinger, J., Perrin, M., Vincent, V., Drugeon, H. Multicenter Evaluation of the MB/BACT System for Susceptibility Testing of Mycobacterium tuberculosis. J Clin Microbiol. 42, 1030-1034 (2004).
- . SENSITITRE MYCOTB Susceptibility Plate: For Mycobacterium Susceptibility Testing. Product Insert. , (2010).
- CLSI. . Susceptiblity Testing of Mycobacteria, Nocardia, and Other Aerobic Actinomycetes; Approved Standard. Document M24-A. , (2003).
- Sullivan, N., Sotos, J., Anhalt, K., Allen, S. Preliminary results from a new TREK Sensititre Mycobacterium tuberculosis MIC plate (MYCOTB). , (2010).
- . VersaTREK MYCO SUSCEPTIBLITY KIT. Product insert. , 7115-7160 (2008).
- Parrish, N. M., Carroll, K. C. The Role of the Clinical Mycobacteriology Laboratory in the Diagnosis and Management of Tuberculosis in Low Prevalance Settings. J Clin Microbiol. , (2010).
- Kam, K. M., Sloutsky, A., Yip, C. W., Bulled, N., Zingol, M., Espinal, M., Kim, S. J. Determination of critical concentrations of second-line anti-tuberculosis drugs with clinical and microbiological relevance. Int J Tubercl Lung Dis. 14, 282-288 (2010).
- Woods, G. L., Warren, N. G., Inderlied, C. B., Murray, P. R., Barron, E. J., Jorgensen, J. H., Landry, M. L., Pfaller, M. A. Susceptibility Test Methods: Mycobacteria, Nocardia, and Other Actinomycetes. Manual of Clinical Microbiology. , 1223-1247 (2007).
- Bergmann, J. S., Woods, G. L. Evaluation of the ESP Culture System II for Testing Susceptibilities of Mycobacterium tuberculosis Isolates to Four Primary Antituberculosis Drugs. J Clin Microbiol. 36, 2940-2943 (1998).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved