A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Electric Field-controlled Directed Migration of Neural Progenitor Cells in 2D and 3D Environments
* These authors contributed equally
In This Article
Summary
This protocol demonstrates methods used to establish 2D and 3D environments in custom-designed electrotactic chambers, which can track cells in vivo/ex vivo using time-lapse recording at the single cell level, in order to investigate galvanotaxis/electrotaxis and other cellular responses to direct current (DC) electric fields (EFs).
Abstract
Endogenous electric fields (EFs) occur naturally in vivo and play a critical role during tissue/organ development and regeneration, including that of the central nervous system1,2. These endogenous EFs are generated by cellular regulation of ionic transport combined with the electrical resistance of cells and tissues. It has been reported that applied EF treatment can promote functional repair of spinal cord injuries in animals and humans3,4. In particular, EF-directed cell migration has been demonstrated in a wide variety of cell types5,6, including neural progenitor cells (NPCs)7,8. Application of direct current (DC) EFs is not a commonly available technique in most laboratories. We have described detailed protocols for the application of DC EFs to cell and tissue cultures previously5,11. Here we present a video demonstration of standard methods based on a calculated field strength to set up 2D and 3D environments for NPCs, and to investigate cellular responses to EF stimulation in both single cell growth conditions in 2D, and the organotypic spinal cord slice in 3D. The spinal cordslice is an ideal recipient tissue for studying NPC ex vivo behaviours, post-transplantation, because the cytoarchitectonic tissue organization is well preserved within these cultures9,10. Additionally, this ex vivo model also allows procedures that are not technically feasible to track cells in vivo using time-lapse recording at the single cell level. It is critically essential to evaluate cell behaviours in not only a 2D environment, but also in a 3D organotypic condition which mimicks the in vivo environment. This system will allow high-resolution imaging using cover glass-based dishes in tissue or organ culture with 3D tracking of single cell migration in vitro and ex vivo and can be an intermediate step before moving onto in vivo paradigms.
Protocol
1. Neural progenitor cell isolation
- Dissect whole brains from E14-16 mice and place in cold DMEM/F12 basal medium. Remove all meninges under an anatomical microscope and transfer brains into a 35 mm Petri dish.
- Use fine forceps to mechanically dissociate brains into tissue fragments and transfer them to a 15 ml tube, then centrifuge samples at 800 rpm for 3 min to remove debris.
- Add DMEM/F12 containing bFGF and EGF and triturate with a 1 ml pipette.
- Pass the cell suspension through a cell strainer to obtain a single cell suspension.
- Plate cells into flasks at 2-5 x 104 cells/ml, then perform a full medium change every 3 days and passage the cells every 6 days.
- After at least 5 passages, digest neurospheres to single cells using trypsin and EDTA and grow on poly-D-lysine/laminin-coated electrotactic chambers (prepared as described below in 2). Use the growing medium containing N2-supplement, bFGF and EGF at all times to maintain properties of NPCs.
2. Preparation of the electrotactic chamber
- Prepare 22 x 11 mm strips of glass by dividing autoclaved 22 x 22 mm no.1 thickness coverslips in half using a diamond pen.
- Create a free-standing glass well of interior dimensions 22 x 10 mm by gluing together four vertically standing 22 x 11 mm strips with high-vacuum silicone grease. Allow the wells to completely dry and harden overnight.
- The following day, attach two 22 x 11 mm glass strips, parallel to each other leaving a gap of 10 mm, to the base of a 100 mm culture dish using silicone grease. Seal off the region between these strips by placing a 22 x 22 mm cover slip at each end, attached to the bottom of the dish with grease on three sides, but not that closest to the centre.
- Place the glass well prepared in step 2.2 onto the cover slips so that the interior walls create a confined space for seeding the cells onto the bottom of the plate. Water-proof all joints with silicone grease. Coat this confined region sequentially with poly-D-lysine then laminin: add 1 ml poly-D-lysine into the chamber and leave for 5 min to allow the poly-D-lysine to bind to the bottom of the plate; wash the chamber with sterile PBS twice, then dilute laminin in sterile PBS to yield 20 μg/ml and use to cover the bottom of the plate. Leave at room temperature over night.
- The following day, harvest cells and prepare 1 ml of suspension containing 1 x 104 cells. Remove laminin from the glass well, allowing it to air dry completely, and replace with 1 ml of cell suspension. Place the dish in the incubator at 37° C for a minimum of 4 hours to allow for attachment.
- Once the cells are sufficiently confluent remove all medium from the chamber. Carefully remove the glass well. Form a roof over the cells by carefully attaching, with silicone grease, an autoclaved 22 x 22 mm glass coverslip bridging between the two 22 x 11 mm strips. Cover the cells with a few drops of medium to avoid drying out.
- Form an isolated medium reservoir at each end of the chamber by creating two watertight silicone grease barriers that run from one edge of the dish to the other, over the chamber roof. Fill the chamber with fresh medium, ensuring a flow-through from one medium reservoir to the other. Return the dish to the incubator for 12 hours to allow for cell recovery.
3. Application of an electric field to the electrotactic chamber
- Prepare a lid to cover the dish by drilling two holes, one positioned over each reservoir of the migration chamber.
- Replace all medium in the chamber with culture medium containing 25 mM HEPES buffer and transfer the dish to the temperature-controlled imaging system. Set up the experimental parameters for time-lapse and multi-position recording. Align the chamber so that the cathode and anode are on the left and right respectively, to ensure that the EF vector runs horizontally as viewed down the microscope and recorded in the imaging system.
- Fill two beakers with Steinberg's solution (58 mM NaCl, 0.67 mM KCl, 0.44 mM Ca(NO3)2.4H20, 1.3 mM MgSO4.7H20 and 4.6 mM Trizma base, pH 7.4). Connect a separate beaker to each medium reservoir using pre-prepared glass bridges (glass tubes ~13 cm long and ~3 mm in diameter, and bent into a U-shape by heating in a Bunsen flame), filled with 2 % (w/v) Steinberg's-agar solution, passing through the holes in the lid. Complete the electric circuit by placing Ag/AgCl electrodes connected to a DC power supply into each beaker of Steinberg's solution.
- Set the voltage dial on the power supply to 0 and switch on. Measure the voltage across the electrotactic chamber while turning up the voltage dial, using a voltage meter, and adjust to suit the experimental requirements.
- Start time-lapse recording. Perform a medium change and readjust the voltage, as required, every hour. Fresh medium, drugs, or chemical agents may be added to the reservoirs as required. When perform medium change, two options can be considered as below:
- Option No.1 - To pause the time-lapse recording temporarily, carefully remove glass bridges from chamber to avoid perturbing cover lid, use a sterilized Pasteur pipette gently replace all medium with fresh medium and put the glass bridges back, then resume the recording.
- Option No.2 - Alternatively, custom made cover lid with 4 holes (two positioned over each reservoir of the migration chamber) can also be used for medium change purpose. Two for glass bridges connection, the other two for changing medium. Option 2 allows continuous recording without interference during medium change.
4. Preparation of the organotypic spinal cord slice
- Dissect lumbar spinal cords of 2 week old C57 BL/6 mice.
- Slice spinal cords into 500 μm thick sections with a McIlwain tissue chopper.
- Separate slices under an anatomical microscope and select slices with intact sagittal/axial spinal cord structure.
- Plate slices in a 35 mm Petri dish containing 30 μl Matrigel and place them as close to the centre as possible and maintain them in a 5 % CO2 incubator at 37 °C for at least 30 min until the Matrigel proteins self-assemble to produce a thin film that covers the surface of the spinal cord slice. It is very important to make sure that Matrigel has been assembled completely, incubate the Petri dish at 37 °C for another 30 min if necessary.
- Add 4-6 ml DMEM/F-12 medium containing 25 mM HEPES buffer and 15-20% fetal calf serum very gently so as to avoid medium flow directly onto the slice. Ensure that the slice is not entirely submerged in medium, leaving the surface of explants well exposed to the air. Change medium twice weekly.
5. Injection of Hoechst 33342 labelled NPCs into the organotypic spinal cord slice
- Prepare the NPC suspension at 1 × 106 cells/μl.
- Pre-incubate the cell suspension in medium with 5 μM Hoechst 33342 for 30 min.
- Use capillary glass tube to microinject 2 μl of suspension into the spinal cord slice slowly under a microscope. Make sure capillary glass tube passes through the matrigel (pink part under microscope) and reach to the inside of spinal cord slice (grey tissue under microscope), remain inside the spinal cord slice for at least 30 seconds to avoid cell suspension run over. Put the petri dish containing spinal cord slice into incubator (37°C 5%CO2) and leave it over night.
- Next day, apply an EF of 500 mV/mm to the spinal cord slice containing Hoechst 33342-labelled NPCs in the electrotactic chamber (using the methods described in 3).
6. Representative Results
When NPCs were exposed to a range of physiological EFs they showed highly directed cell migration towards the cathode (Figure 1). The same observation was also made at a single cell level on the organotypic spinal cord slice ex vivo model, a 3D environment mimicking in vivo conditions (Figure 2).
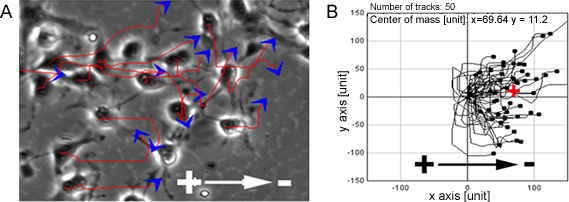
Figure 1. NPCs show directed migration in EFs. PCs showed highly directed migration towards the cathode when exposed to EFs, red lines and blue arrows represent trajectories and direction of cell movement (A). B shows migration paths of NPCs. Bar: 50 μm.
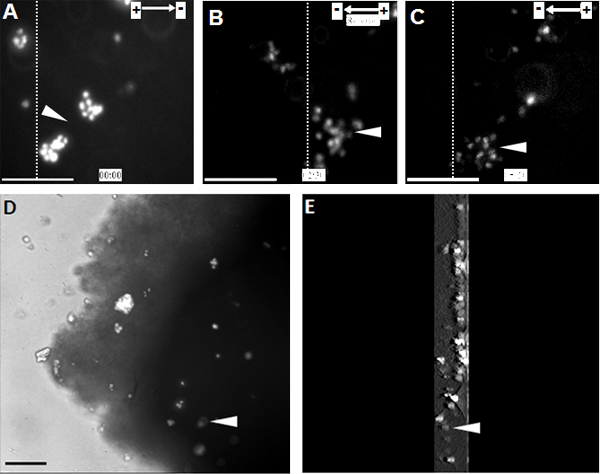
Figure 2. Transplanted NPCs show directed migration towards the cathode in the organotypic spinal cord slice. (A) NPCs labeled with Hoechst 33342 were transplanted into the organotypic spinal cord slice at the starting point of the EF treatment. NPCs migrated directionally towards the cathode for 2.5 hours, at which point the EF polarity was reversed (B). Altering EF polarity triggered a sharp reversal of electrotaxis towards the new cathode (C). (D) Image of transplanted NPCs within the spinal cord slice at the end of the time-lapse recording. (E) A 3D reconstruction of transplanted NPCs within the spinal cord slice. 3D scanning sections were 300 μm in thickness, starting from the middle and ending at the bottom of the slice. Dotted lines indicate the relative positions of the same population of transplanted cells at the beginning, reversal, and end points of the EF treatment (A - C, respectively). Arrow heads indicate the same population of Hoechst 33342 labelled NPCs. Bar: 50 μm.
Discussion
The protocols we use are based on previous studies5,11. Using these methods, stable culture and electric current conditions can be maintained while applying an EF via agar bridges, Steinberg's solution, and Ag/AgCl electrodes, to cells or slices cultured in custom-designed electrotactic chambers of standardised and precise dimensions. The depth of chambers can be adjusted to accommodate for different sample thicknesses11, and in the case of cells, chamber size can be modified to accommodate however ...
Disclosures
We have nothing to disclose.
Acknowledgements
This work was supported by the Royal Society URF grant UF051616, UK and the European Research Council StG grant 243261 to BS. The work in MZ lab is also supported by a California Institute of Regenerative Medicine grant RB1-01417.
Materials
| Name | Company | Catalog Number | Comments |
| FGF-basic Recombinant Human | Invitrogen | PHG0026 | 20 ng/mL |
| EGF Recombinant Human | Invitrogen | PHG0311 | 20 ng/mL |
| N2-Supplement (100X) liquid | Invitrogen | 02048 | |
| DMEM/F12 medium (high glucose) | Invitrogen | 31330-095 | |
| Poly-D-Lysine | EMD Millipore | A-003-E | |
| Natural mouse Laminin | Invitrogen | 23017-015 | |
| Growth factor reduced Basement Membrane Matrix (Matrigel) | BD Biosciences | 354230 | |
| HEPES buffer | GIBCO, by Life Technologies | 15630 | |
| McIlwain tissue chopper | The Mickle Laboratory Engineering Co Ltd | TC752-PD | |
| Dow Corning high-vacuum silicone grease | Sigma-Aldrich | Z273554 |
References
- Huttenlocher, A., Horwitz, A. R. Wound healing with electric potential. N. Engl. J. Med. 356, 303-303 (2007).
- McCaig, C. D., Rajnicek, A. M., Song, B. Controlling cell behavior electrically: current views and future potential. Physiol. Rev. 85, 943-943 (2005).
- Borgens, R. B., Jaffe, L. F., Cohen, M. J. Large and persistent electrical currents enter the transected lamprey spinal cord. PNAS. 77, 1209-1209 (1980).
- Shapiro, S., Borgens, R., Pascuzzi, R. Oscillating field stimulation for complete spinal cord injury in humans: a phase 1 trial. J. Neurosurg. Spine. 2, 3-3 (2005).
- Zhao, M., Song, B., Pu, J. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 442, 457-457 (2006).
- Yao, L., Shanley, L., McCaig, C. Small applied electric fields guide migration of hippocampal neurons. J. Cell. Physiol. 216, 527-527 (2008).
- Li, L., El-Hayek, Y. H., Liu, B. Direct-current electrical field guides neuronal stem/progenitor cell migration. Stem Cells. 26, 2193-2193 (2008).
- Meng, X., Arocena, M., Penninger, J. PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Exp. Neurol. 227, 210-210 (2011).
- Bonnici, B., Kapfhammer, J. P. Bone marrow stromal cells promote neurite extension in organotypic spinal cord slice: significance for cell transplantation therapy. Neurorehabil. Neural Repair. 22, 447-447 (2008).
- Shichinohe, H., Kuroda, S., Tsuji, S. Bone marrow stromal cells promote neurite extension in organotypic spinal cord slice: significance for cell transplantation therapy. Neurorehabil. Neural Repair. 22, 447-447 (2008).
- Song, B., Gu, Y., Pu, j. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nature Protocol. 2, 1479-1479 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved