A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Isolation and Primary Culture of Rat Hepatic Cells
In This Article
Summary
Primary hepatocytes provide a valuable tool to evaluate biochemical, molecular, and metabolic functions in a physiologically relevant experimental system. We describe a reliable protocol for rat in situ liver perfusion, which consistently generates viable hepatocytes up to 1.0 × 108 cells per preparation with cell viability between 88 ~ 96%.
Abstract
Primary hepatocyte culture is a valuable tool that has been extensively used in basic research of liver function, disease, pathophysiology, pharmacology and other related subjects. The method based on two-step collagenase perfusion for isolation of intact hepatocytes was first introduced by Berry and Friend in 1969 1 and, since then, has undergone many modifications. The most commonly used technique was described by Seglenin 1976 2. Essentially, hepatocytes are dissociated from anesthetized adult rats by a non-recirculating collagenase perfusion through the portal vein. The isolated cells are then filtered through a 100 μm pore size mesh nylon filter, and cultured onto plates. After 4-hour culture, the medium is replaced with serum-containing or serum-free medium, e.g. HepatoZYME-SFM, for additional time to culture. These procedures require surgical and sterile culture steps that can be better demonstrated by video than by text. Here, we document the detailed steps for these procedures by both video and written protocol, which allow consistently in the generation of viable hepatocytes in large numbers.
Protocol
1. Preparation
All buffers are freshly prepared using sterile technique and filter sterilized using a Corning 0.22 μm filter.
- Prepare Perfusion buffer I by adding the following to Hank's Balanced Salt Solution (HBSS, without Ca2+ and Mg2+, see Table 1): Mg2+ (MgCl2) to 0.9 mM, EDTA to 0.5 mM, and HEPES to 25 mM.
- Prepare Perfusion buffer II by adding the following to HBSS (with Ca2+ and Mg2+, see Table 1): HEPES to 25 mM.
- Prepare Perfusion buffer II plus collagenaseII: Dissolve collagenase II (1000 U) with 300 ml Perfusion buffer II and keep the solution warm in water-bath before perfusion. This solution should be used within 30 min because the activity of collagenase II decreased with the time.
- Prepare William's complete Medium: Add the following to Williams' Medium E: L-glutamine to 2 mM, fetal bovine serum (FBS) to 5%; insulin to 100 nM, dexamethasone to 100 nM, penicillin to 100 IU/ml and streptomycin to 100 mg/ml.
- These buffers should be warmed for 30 minutes in the water bath at 42 °C, an optimal temperature corresponding to an outlet temperature at the cannula of 37 °C.
2. Rat Perfusion for Liver Isolation
- The perfusion system consists of the pump, autoclavable silastic tubing and a water bath (see Figure 1). Pre-set the flow rate of the peristaltic perfusion pump to 10 ml/min.
- Anesthetize an adult rat (300 g body weight) with intraperitoneal (ip) ketamine (87 mg/kg body weight) plus xylazine (13 mg/kg). Depth of anesthesia should be monitored by toe pinch. When the rat no longer responds to noxious stimulus, shave abdominal hair and prep the abdomen with betadine and ethanol. Enter through a midline incision.
- Expose the hepatic portal vein by carefully moving the viscera to the right outside of the abdominal cavity, and insert an 18-gauge angiocath into the hepatic portal vein (see Figure 1).
- Connect the perfusate tubing to the needle and initiate the infusion in situ at a low flow rate (10 ml/min) with pre-warmed (37 °C) Perfusion Buffer I.
- If performed properly, the liver should instantly begin to blanch. Once successful cannulation is confirmed, make a cut at inferior vena cava (IVC) to allow efflux (Figure 1). A further test for successful cannulation can be performed by applying light pressure with sterile swab on the IVC; all lobes of the liver should quickly begin to swell.
- Increase the rate of flow to 25 ml/min. The liver should become pale in color.
- Switch the perfusion solution to Perfusion buffer II plus collagenase II with no interruption of flow for an additional 6 minutes.
- Periodically (5-10 times during digestion) apply pressure with swab to the IVC for 5-second intervals. The liver will swell, leading to enhanced hepatic cell dissociation, in turn reducing total digestion time, and increasing final yield.
- After collagenase perfusion, liver should begin to look mushy. Dissect the liver free, place in a pre-chilled sterile beaker with 20 ml William's complete Medium, and then take it to tissue cell culture hood.
3. Hepatocyte Cell Isolation
- Within the cell culture hood, use a cell scraper to gently disperse the cells into William's complete Medium within a sterile Petri dish.
- Filter the cell dispersion through a 100 μm pore size cell strainer into a 50 ml Conical tube in order to remove connective tissues and undigested tissue fragments.
- Suspend cells in 40 ml William's complete Medium and centrifuge at 50 x g for 3 min at 4 °C.
- Aspirate the supernatant, and gently re-suspend cells in 40 ml cold William's complete Medium to wash cells. Repeat centrifugation.
- Aspirate the supernatant, and gently re-suspend cells with 25 ml William's complete Medium. Add 25 ml 90% Percoll solution in PBS into the tube and gently mix.
- Centrifuge at 200 x g for 10 min at 4 °C. Aspirate the dead cells from the top of the gradient because the viable cells remain at the bottom of the Percoll gradient.
- Suspend the cell pellet in 30 ml warm William's complete Medium, and then repeat centrifugation and re-suspend the cell pellet in 20 ml warm William's complete Medium.
- Count the cells within the cell suspension using a hemocytometer and determine cell viability by trypan blue staining.
4. Hepatocyte Culture
- Dilute cells with warm William's complete Medium to preferred concentration, e.g. 2.5 x 105 cells/ml. Plate cells at a desired volume on cell culture plates, e.g. 5 x 105 cells / 2 ml / well, 6 wells/plate; or 2.5 x 105 cells / 1.5 ml / well, 12 wells / plate. Un-coated plate is fine for hepatic cell cultures.
- In a typical prep with > 85% viable cells, the cell density should reach approximately 60-70% confluence, which allows for cell-cell contact while maintaining sufficient space for the hepatocytes to grow to their full cell size and yield a final confluence of 90-95%.
- In order to form an even monolayer of hepatocytes, in other words, to minimize the tendency for cells to aggregate in the central area of the well (most likely due to the air-flow inside of cell incubator), allow the plates to remain inside the cell culture hood for 30 minutes before placing them in a incubator.
- Culture the cells at 37 °C in a humidified atmosphere of 95% air and 5 % CO2. After 4-h culture, the cells could either remain in the same serum-containing medium or replace the medium with serum-free medium, e.g. HepatoZYME-SFM (see Table 1). The serum-free medium helps to maintain cell morphology with no adverse effects from hormones when a serum-free medium is used.
- Allow cells to recover and grow at least overnight prior to experimentation. We recommend to use the cells for test within 24 hr because this may help preserve the function of critical enzymes (e.g., P450s).
- Replace the growth medium at 2 day intervals, if needed.
5. Representative Results
The conditions described regularly generate cell harvests of 1.0 x 108 cells per preparation from one rat liver. The viability of the hepatocytes measured by trypan blue exclusion was consistently within the range of 88 ~96%.
As depicted in Figure 2, the hepatocytes aggregate and form cluster after 4 hr seeding. Most isolated cells flatten and spread in typical monolayer growth. By 24 hr, the edges of cells are defined, surface of cells is fairly smooth, and lipid droplets are visible. The cells have one to three nucleoli, which are round, located at the center of the cells, and the nuclei display consistent size among cells (Figure 2).
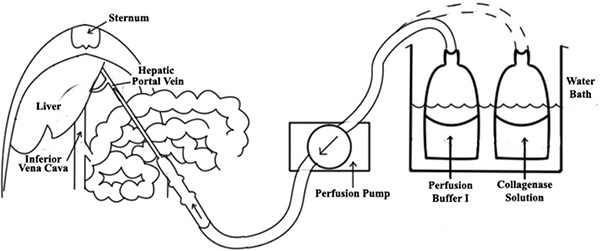
Figure 1. A diagram of liver perfusion. An 18-gauge angiocath is inserted into the portal vein of liver, then a perfusate tubing is connected to the needle. Once successful cannulation is confirmed, make a cut at inferior vena cava (IVC) to allow efflux.
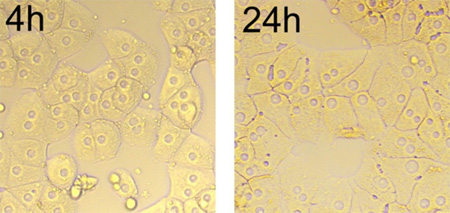
Figure 2. Morphology of cultured hepatocytes over time (4 hr to 24 hr), magnification x 200. After cultured for 24 hr, the cells spread in typical monolayer growth, and the junctions between the cells are linear.
| Name of the reagent | Company | Catalog number |
| HBSS (without Ca2+, and Mg2+) | Invitrogen | 14174 |
| HBSS (with Ca2+, and Mg2+) | Invitrogen | 14025 |
| Williams's Medium E | Invitrogen | 12551-032 |
| Collegenase II | Worthington | LS004176 |
| Cell strainer (100 μm) | BD | 352360 |
| HepatoZYME-SFM | Invitrogen | 17705 |
| Percoll | Sigma | P4937 |
| Angiocath (18-gauge) | BD | 381705 |
Table 1.
Discussion
Primary culture of hepatocytes is an in vitro model widely used to study various aspects of liver physiology and pathology. For example, primary culture is used to assess the expression and function of drug-metabolizing enzymes including cytochromes P450, drug metabolism, drug-drug interactions, and the mechanisms of cytotoxicity and genotoxicity 3-7. The protocol describing isolation and culture of rat hepatic cells is adapted from the previous reports of Aiken et al. 8, a...
Disclosures
No conflicts of interest declared.
Acknowledgements
The authors would like to thank Mr. Josh Basford and Dr. Xiao-min Li for technical assistance. This work was supported in part by NIH grants (DK70992 and DK92779 to M.L.).
References
- Berry, M. N., Friend, D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J. Cell Biol. 43, 506-520 (1969).
- Seglen, P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 13, 29-83 (1976).
- Saito, K., Kobayashi, K., Mizuno, Y., Fukuchi, Y., Furihata, T., Chiba, K., Schmidt, M., Schmitz, H. J., Baumgart, A., Guedon, D. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonists induce constitutive androstane receptor (CAR) and cytochrome P450 2B in rat primary hepatocytes. Drug Metab. Pharmacokinet. 25, 108-111 (2010).
- Gomez-Lechon, M. J., Donato, M. T., Castell, J. V., Jover, R. Human hepatocytes in primary culture: the choice to investigate drug metabolism in man. Curr. Drug Metab. 5, 443-462 (2004).
- Goncalves, L. A., Vigario, A. M., Penha-Goncalves, C. Improved isolation of murine hepatocytes for in vitro malaria liver stage studies. Malar. J. 6, 169 (2007).
- Bu, S. Y., Mashek, D. G. Hepatic long-chain acyl-CoA synthetase 5 mediates fatty acid channeling between anabolic and catabolic pathways. J. Lipid Res. 51, 3270-3280 (2010).
- Aiken, J., Cima, L., Schloo, B., Mooney, D., Johnson, L., Langer, R., Vacanti, J. P. Studies in rat liver perfusion for optimal harvest of hepatocytes. J. Pediatr. Surg. 25, 140-144 (1990).
- Jauregui, H. O., McMillan, P. N., Hevey, K., Naik, S. A quantitative analysis of lectin binding to adult rat hepatocyte cell surfaces. In Vitro Cell. 24, 401-412 (1988).
- Klaunig, J. E., Goldblatt, P. J., Hinton, D. E., Lipsky, M. M., Trump, B. F. Mouse liver cell culture. II. Primary culture. In Vitro. 17, 926-934 (1981).
- Hay, R. J. Operator-induced contamination in cell culture systems. Dev. Biol. Stand. 75, 193-204 (1991).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved