A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Graphene Coatings for Biomedical Implants
In This Article
Summary
Graphene offers potential as a coating material for biomedical implants. In this study we demonstrate a method for coating nitinol alloys with nanometer thick layers of graphene and determine how graphene may influence implant response.
Abstract
Atomically smooth graphene as a surface coating has potential to improve implant properties. This demonstrates a method for coating nitinol alloys with nanometer thick layers of graphene for applications as a stent material. Graphene was grown on copper substrates via chemical vapor deposition and then transferred onto nitinol substrates. In order to understand how the graphene coating could change biological response, cell viability of rat aortic endothelial cells and rat aortic smooth muscle cells was investigated. Moreover, the effect of graphene-coatings on cell adhesion and morphology was examined with fluorescent confocal microscopy. Cells were stained for actin and nuclei, and there were noticeable differences between pristine nitinol samples compared to graphene-coated samples. Total actin expression from rat aortic smooth muscle cells was found using western blot. Protein adsorption characteristics, an indicator for potential thrombogenicity, were determined for serum albumin and fibrinogen with gel electrophoresis. Moreover, the transfer of charge from fibrinogen to substrate was deduced using Raman spectroscopy. It was found that graphene coating on nitinol substrates met the functional requirements for a stent material and improved the biological response compared to uncoated nitinol. Thus, graphene-coated nitinol is a viable candidate for a stent material.
Introduction
The past three decades have witnessed discovery of novel materials-based therapies and devices for disease treatments and diagnostics. Novel alloy materials such as nitinol (NiTi) and stainless steel are often used in biomedical implant manufacturing due to their superior mechanical properties.1-3 However, numerous challenges remain due to exogenous material cytotoxicity, bio- and hemo-compatibility. The metallic nature of these alloys results in poor bio- and hemocompatibility due to metal leaching, lack of cell adhesion, proliferation, and thrombosis when it comes in contact with flowing blood (such as catheters, blood vessel grafts, vascular stents, artificial heart valves etc.). 1, 4, 5 The interaction of proteins or living cells with the implant surface can lead to a strong immunological response and the ensuing cascade of biochemical reactions may adversely affect the device functionality. Therefore, it is pertinent to achieve control over the interactions between biomedical implants and its surrounding biological environment. Surface modification is often employed to reduce or prevent the adverse physiological response originating from the implant material. An ideal surface coating is expected to have high adhesion strength, chemical inertness, high smoothness, and good hemo- and biocompatibility. Previously, numerous materials including diamond-like carbon (DLC), SiC, TiN, TiO2 and many polymer materials have been tested as bio-compatible implant surface coatings. 1, 6-23 However, these materials are still unable to meet all of the functional criteria for a suitable implant surface coating.
The discovery of atom thick layer of sp2 carbon, known as graphene, has opened doors for the development of novel multifunctional materials. Graphene is expected to be an ideal candidate for implant surface coating since it is chemically inert, atomically smooth and highly durable. In this Letter, we investigate the viability of graphene as a surface coating for biomedical implants. Our studies show that the graphene coated nitinol (Gr-NiTi) meets all of the functional criteria, and additionally supports excellent smooth muscle and endothelial cell growth leading to better cell proliferation. We also find that the serum albumin adsorption on Gr-NiTi is higher than fibrinogen. Importantly, (i) our detailed spectroscopic measurements confirmed the lack of charge transfer between graphene and fibrinogen suggesting that graphene coating inhibits platelet activation by implants, (ii) graphene coatings do not exhibit any significant in vitro toxicity for endothelial and smooth muscle cell lines confirming their biocompatibility, and (iii) graphene coatings are chemically inert, durable and impermeable in flowing blood environment. These hemo-and biocompatible properties, along with high strength, chemical inertness and durability, render graphene coatings as an ideal surface coating.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Graphene-coating of NiTi
- The graphene samples used in this study were grown on copper (Cu) substrates using the chemical vapor deposition technique, and subsequently transferred to 4.5 mm2NiTi substrates.
- Cu foils (1 cm x 1 cm) were placed in a 1 in. quartz tube furnace and heated to 1,000 °C in the presence of 50 sccm of H2 and 450 sccm of Ar.
- Next, methane (1 and 4 sccm) was introduced into the furnace at different flow rates for 20-30 min. The samples were finally cooled to room temperature under flowing H2, Ar and CH4.
- Next, the Cu foils were spin-coated with PMMA (diluted with 4% anisole) at 4,000 rpm followed by a heat treatment for 5 min at 150 °C. Graphene attached to PMMA layer was obtained by etching the Cu foil using Transene Inc., CE-100 etchant, and subsequent rinsing in 10% HCl and de-ionized water for 10 min.
- The samples were transferred to NiTi substrates (4.5 mm2) and annealed at 450 °C in Ar (300 sccm) and H2 (700 sccm) for 2 hr to remove the PMMA. Finally, the substrates were washed with acetone to dissolve the residual PMMA to obtain the Gr-NiTi sample. A Dilor XY triple grating monochromator was used for the micro-Raman characterization (using 100X objective) of all Gr-NiTisamples with the 514.5 nm excitation from an Ar+ ion laser.
2. In Vitro Toxicity of Gr-NiTi
Rat aortic endothelial cells (Cell application Inc.,) were cultured on a gelatin coated 8 chambers slide. For testing the cell growth, pristine and Gr-NiTisubstrates were placed in wells without any gelatin coating. Scanning electron microscopy images were obtained using a Hitachi S-4800 SEM. Additionally, rat aortic smooth muscle cells were also grown in CellBind 96-well plates as a control group (Corning) in Dulbecco's Modified Eagle Medium (ATCC).
- For testing cell viability, cells (both endothelial and smooth muscle cells) were seeded at 105 cells/well in wells containing pristine NiTi, 1 sccm or 4 sccm Gr-NiTi substrates, where the stated sccm corresponds to the methane flow used in the CVD growth of graphene. Cells were grown for the desired time period in an incubator at 37 °C and 5% CO2, exchanging media every other day.
- At the end time point, media was removed and fresh media containing 0.5 mg/ml thiazolyl blue tetrazolium bromide (or MTT obtained from Sigma) was added to each well. Cells were then incubated for an additional 3 hr. For the MTS assay, media was removed at the final time point and replaced with 120 μl of MTS working solution (Cell Tier 96 aqueous, Promega) and incubated for 3 hr.
- Next, media was gently removed and 100 ml of dimethylsulfoxide (Sigma) was added to each well. After allowing 10 min for the MTT crystals to dissolve, the solution was transferred to another well plate. For the MTS assay, no dimethylsulfoxide was added to the wells. Well contents were moved to a new plate.
- Absorbance was read at 490 nm and the percent viability was determined by normalizing absorbance to the average absorbance of the pristine NiTi sample. At least five repeats were done for each sample type.
3. Confocal Microscopy Studies of Cell Morphology
- For confocal imaging of rat aortic smooth muscle cells, substrates were placed in an 8-chamber slide (Thermo Scientific). The cells were seeded at 25,000 cells/chamber and incubated for 3 days at 37 °C and 5% CO2.
- Cells were fixed on the substrate with 4% formaldehyde in phosphate buffered saline for 20 min.
- Permeabilized with 0.1% Triton-X for 1 min.
- Actin was stained with Alexa Fluor 488 phalloidin (Life Technologies). 100 μl of alexafluor 488 phalloidin at 200 units/ml in methanol was added to 1.9 ml of phosphate buffered saline. Cells were stained with 250 μl of alexafluor 488 phalloidin for 45 min and then washed twice with phosphate buffered saline.
- Nuclei were mounted with VectaShield fluorescent mounting medium containing DAPI (Vector Laboratories). Confocal images were collected using a Nikon Confocal TI. A chamber seeded with cells without any substrate was used as a control.
4. Protein Adsorption Studies
- Substrate dimensions were measured with calipers before starting the protein adsorption experiments. Three measurements were taken for each side of the approximately square samples and averaged to get the length and width.
- Each sample, pristine NiTi, 1sccm and 4sccm Gr-NiTiwere incubated with 1 mg/ml albumin in phosphate buffered saline (PBS) or 1 mg/ml fibrinogen in PBS at room temperature for 3 hr.
- Alike samples were combined in a microcentrifuge tube with 200 μl of reducing sample buffer and boiled for 5 min.
- Samples were then diluted in a Tris/Glycine/SDS buffer (Bio-Rad) and run through a 4-15% Tris polyacrylamide electrophoresis gel (Bio-Rad) at 90 V for 100 min.
- Gels were then stained with SYPRO Red. Dilute SYPRO Red (Life Technologies) stock solution at 1:5,000 in 7.5 v/v% acetic acid. Stain gels for 60 min.
- Image gels using a Flourchem SP (Alpha Innotech).Fluorescent intensity was quantified using ImageJ software. Fluorescent intensity from each sample was normalized by the total area of substrate and fibrinogen adsorption was compared to albumin adsorption.
5. Western Blotting for Protein Expression
- Western blot was performed to analyze total actin in rat aortic smooth muscle cells. Cells were seeded at 10,000 cells/substrate in a 96-well plate.
- Cells were grown for three days before removing media. Total protein was extracted using RIPA buffer and a standard BCA assay (Lamda) was performed to quantify total protein.
- Samples were diluted to the same concentration in RIPA and then boiled in a reducing sample buffer for 5 min.
- Proteins were separated by a 4-15% Tris polyacrylamide gel via electrophoresis at 90 V for 100 min. A kaleidoscope protein standard (Bio-Rad) was used to assess protein molecular weight.
- Proteins were transferred to a PVDF membrane and blocked with a 1% non-fat dry milk (Bio-Rad) solution.
- Total actin was tagged with a rabbit anti-rat actin antibody (Sigma). A BM chemiluminescent kit (Roche) was used to detect the primary antibody. Membranes were imaged using FlourChem SP imaging equipment and fluorescent intensity was measured using ImageJ software. Fluorescent intensity was normalized by comparing to the pristine NiTisample.
Access restricted. Please log in or start a trial to view this content.
Results
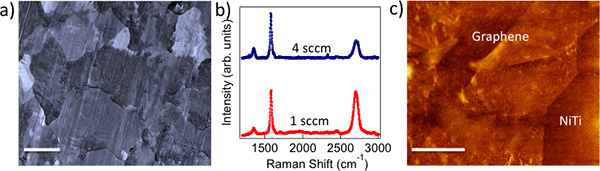
Figure 1. a) CVD grown polycrystalline graphene on Cu foils mimics the metal crystal grains (scale bar: 10 μm). b) Raman spectrum of 1 sccm (4 sccm) graphene shows intense (relatively weaker) G' band indicating monolayer (few layer) nature of as-prepared graphene. c) AFM image of graphene transferred on to NiTi shows a roughness of ~5 nm. Scale bar = 500 nm.
Access restricted. Please log in or start a trial to view this content.
Discussion
Biocompatibility and cytotoxicity: The chemical vapor deposition (CVD) method yielded polycrystalline graphene samples that mimicked Cu crystal grains, as shown in Figure 1a. We employed Raman spectroscopy to confirm the presence of monolayer (few layer) graphene on 1 sccm (4 sccm) samples (see Figure 1b). Clearly, 1 sccm (4 sccm) samples exhibit intense (relatively weaker) G' band indicative of monolayer (few layer) graphene. Figure 1c shows an atomic forc...
Access restricted. Please log in or start a trial to view this content.
Disclosures
No conflicts of interest declared.
Materials
| Name | Company | Catalog Number | Comments |
| Reagent | |||
| Dulbecco's Modified Eagle Medium | ATCC | 30-2002 | |
| Thiazolyl blue tetrazolium bromide | Sigma-Aldrich | M2128 | |
| CellTiter 96 Aqueous One solution cell proliferation assay (MTS) | Promega | G3582 | |
| Dimethyl sulfoxide | Sigma-Aldrich | D8418 | |
| 36.5% formaldehyde | Sigma-Aldrich | F8775 | |
| Triton X-100 | Sigma-Aldrich | T8787 | |
| Alexafluor 488 phalloidin | Life Technologies | A12379 | |
| VECTASHIELD mounting medium with DAPI | Vector Laboratories | H-1200 | |
| Human serum albumin | Sigma-Aldrich | A9511 | |
| Human fibrinogen | |||
| Tris/Glycine/SDS | Bio-Rad | 161-0732 | |
| Ready Gel Tris-HCl Gel | Bio-Rad | 161-1158 | |
| Acetic acid | Sigma-Aldrich | 45726 | |
| SYPRO Red | Life Technologies | S-6653 | |
| Protein low BCA assay | Lamda Biotech | G1003 | |
| Precision Plus Protein Kaleidoscope Standard | Bio-Rad | 161-0375 | |
| Immun-Blot PVDF membrane | Bio-Rad | 162-0177 | |
| Blotting grade blocker non-fat dry milk | Bio-Rad | 170-6404XTU | |
| Anti-actin antibody produced in rabbit | Sigma-Aldrich | A2066 | |
| BM Chemiluminescence Western Blotting kit (mouse/rabbit) | Roche Applied Science | 11520709001 | |
| RIPA buffer | Sigma-Aldrich | R0278 | |
| NiTi (51% Ni, 49% Ti) | Alfa-Aesar | 44953 | |
| Equipment | |||
| Horiba JobinYvon | Raman spectrometer | Dilor XY 98 | |
| Nikon | Confocal microscope | Eclipse TI microscope | |
| Thermoscientific | Plate reader | ||
| Bio-Rad | Power supply | 164-5050 | PowerPac basic power supply |
| Bio-Rad | Electrophoresis cell | 165-8004 | Mini-PROTEAN tetra cell |
| Bio-Rad | Gel holder cassette | 170-3931 | Mini gel holder cassette |
References
- Roy, R. K., Lee, K. -R. Biomedical applications of diamond-like carbon coatings: A review. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 83 B (1), 72-84 (2007).
- Shah, A. K., Sinha, R. K., Hickok, N. J., Tuan, R. S. High-resolution morphometric analysis of human osteoblastic cell adhesion on clinically relevant orthopedic alloys. Bone. 24 (5), 499-506 (1999).
- Huang, N., Yang, P., Leng, Y. X., Chen, J. Y., Sun, H., Wang, J., Wang, G. J., Ding, P. D., Xi, T. F., Leng, Y. Hemocompatibility of titanium oxide films. Biomaterials. 24 (13), 2177-2187 (2003).
- Gutensohn, K., Beythien, C., Bau, J., Fenner, T., Grewe, P., Koester, R., Padmanaban, K., Kuehnl, P. In vitro analyses of diamond-like carbon coated stents: Reduction of metal ion release, platelet activation, and thrombogenicity. Thrombosis Research. 99 (6), 577-585 (2000).
- Gillespie, W. J., Frampton, C. M. A., Henderson, R. J., Ryan, P. M. The Incidence of Cancer Following Total Hip-Replacement. Journal of Bone and Joint Surgery-British Volume. 70 (4), 539-542 (1988).
- Sperling, C., Schweiss, R. B., Streller, U., Werner, C. In vitro hemocompatibility of self-assembled monolayers displaying various functional groups. Biomaterials. 26 (33), 6547-6557 (2005).
- Mikhalovska, L. I., Santin, M., Denyer, S. P., Lloyd, A. W., Teer, D. G., Field, S., Mikhalovsky, S. Fibrinogen adsorption and platelet adhesion to metal and carbon coatings. Thrombosis and Haemostasis. 92 (5), 1032-1039 (2004).
- Airoldi, F., Colombo, A., Tavano, D., Stankovic, G., Klugmann, S., Paolillo, V., Bonizzoni, E., Briguori, C., Carlino, M., Montorfano, M., Liistro, F., Castelli, A., Ferrari, A., Sgura, F., Mario, C. D. i Comparison of diamond-like carbon-coated stents versus uncoated stainless steel stents in coronary artery disease. American Journal of Cardiology. 93 (4), 474-477 (2004).
- Allen, M., Myer, B., Rushton, N. In vitro and in vivo investigations into the biocompatibility of diamond-like carbon (DLC) coatings for orthopedic applications. Journal of Biomedical Materials Research. 58 (3), 319-328 (2001).
- Butter, R., Allen, M., Chandra, L., Lettington, A. H., Rushton, N. In-vitro Studies of DLC Coatings with Silicon Intermediate Layer. Diamond and Related Materials. 4 (5-6), 857-861 (1995).
- Dearnaley, G., Arps, J. H. Biomedical applications of diamond-like carbon (DLC) coatings: A review. Surface & Coatings Technology. 200 (7), 2518-2524 (2005).
- Dorner-Reisel, A., Schurer, C., Nischan, C., Seidel, O., Muller, E. Diamond-like carbon: alteration of the biological acceptance due to Ca-O incorporation. Thin Solid Films. 420, 263-268 (2002).
- Dowling, D. P., Kola, P. V., Donnelly, K., Kelly, T. C., Brumitt, K., Lloyd, L., Eloy, R., Therin, M., Weill, N. Evaluation of diamond-like carbon-coated orthopaedic implants. Diamond and Related Materials. 6 (2-4), 390-393 (1997).
- Grill, A. Diamond-like carbon coatings as biocompatible materials - an overview. Diamond and Related Materials. 12 (2), 166-170 (2003).
- Hauert, R. A review of modified DLC coatings for biological applications. Diamond and Related Materials. 12 (3-7), 583-589 (2003).
- Windecker, S., Mayer, I., De Pasquale, G., Maier, W., Dirsch, O., De Groot, P., Wu, Y. P., Noll, G., Leskosek, B., Meier, B., Hess, O. M. Working Grp Novel Surface, C., Stent coating with titanium-nitride-oxide for reduction of neointimal hyperplasia. Circulation. 104 (8), 928-933 (2001).
- Zhang, F., Zheng, Z. H., Chen, Y., Liu, X. G., Chen, A. Q., Jiang, Z. B. In vivo investigation of blood compatibility of titanium oxide films. Journal of Biomedical Materials Research. 42 (1), 128-133 (1998).
- Bolz, A., Schaldach, M. Artificial-Heart Valves - Improved Blood Compatibility By PECVD a-SiC-H COATING. Artificial Organs. 14 (4), 260-269 (1990).
- Haude, M., Konorza, T. F. M., Kalnins, U., Erglis, A., Saunamaki, K., Glogar, H. D., Grube, E., Gil, R., Serra, A., Richardt, H. G., Sick, P., Erbel, R., Invest, C. T. Heparin-coated stent placement for the treatment of stenoses in small coronary arteries of symptomatic patients. Circulation. 107 (9), 1265-1270 (2003).
- Suggs, L. J., Shive, M. S., Garcia, C. A., Anderson, J. M., Mikos, A. G. In vitro cytotoxicity and in vivo biocompatibility of poly(propylene fumarate-co-ethylene glycol) hydrogels. Journal of Biomedical Materials Research. 46 (1), 22-32 (1999).
- Clarotti, G., Schue, F., Sledz, J., Benaoumar, A. A., Geckeler, K. E., Orsetti, A., Paleirac, G. Modification of the biocompatible and haemocompatible properties of polymer substrates by plasma-deposited fluorocarbon coatings. Biomaterials. 13 (12), 832-840 (1992).
- Gombotz, W. R., Guanghui, W., Horbett, T. A., Hoffman, A. S. Protein adsorption to poly(ethylene oxide) surfaces. Journal of Biomedical Materials Research. 25 (12), 1547-1562 (1991).
- Ishihara, K., Fukumoto, K., Iwasaki, Y., Nakabayashi, N. Modification of polysulfone with phospholipid polymer for improvement of the blood compatibility. Part 2. Protein adsorption and platelet adhesion. Biomaterials. 20 (17), 1553-1559 (1999).
- Jung, N., Kim, B., Crowther, A. C., Kim, N., Nuckolls, C., Brus, L. Optical Reflectivity and Raman Scattering in Few-Layer-Thick Graphene Highly Doped by K and Rb. ACS Nano. 5 (7), 5708-5716 (2011).
- Rao, A. M., Eklund, P. C., Bandow, S., Thess, A., Smalley, R. E. Evidence for charge transfer in doped carbon nanotube bundles from Raman scattering. Nature. 388 (6639), 257-259 (1997).
- Bunch, J. S., Verbridge, S. S., Alden, J. S., vander Zande, A. M., Parpia, J. M., Craighead, H. G., McEuen, P. L. Impermeable atomic membranes from graphene sheets. Nano Letters. 8 (8), 2458-2462 (2008).
- Chen, S., Brown, L., Levendorf, M., Cai, W., Ju, S. -Y., Edgeworth, J., Li, X., Magnuson, C. W., Velamakanni, A., Piner, R. D., Kang, J., Park, J., Ruoff, R. S. Oxidation Resistance of Graphene-Coated Cu and Cu/Ni Alloy. Acs Nano. 5 (2), 1321-1327 (2011).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved