Method Article
Establishing Fungal Entomopathogens as Endophytes: Towards Endophytic Biological Control
In This Article
Summary
This protocol demonstrates two inoculation methods to introduce the fungal entomopathogen Beauveria bassiana as an endophyte in the common bean (Phaseolus vulgaris), in preparation for subsequent evaluations of endophytic biological control.
Abstract
Beauveria bassiana is a fungal entomopathogen with the ability to colonize plants endophytically. As an endophyte, B. bassiana may play a role in protecting plants from herbivory and disease. This protocol demonstrates two inoculation methods to establish B. bassiana endophytically in the common bean (Phaseolus vulgaris), in preparation for subsequent evaluations of endophytic biological control. Plants are grown from surface-sterilized seeds for two weeks before receiving a B. bassiana treatment of 108 conidia/ml (or water) applied either as a foliar spray or a soil drench. Two weeks later, the plants are harvested and their leaves, stems and roots are sampled to evaluate endophytic fungal colonization. For this, samples are individually surface sterilized, cut into multiple sections, and incubated in potato dextrose agar media for 20 days. The media is inspected every 2-3 days to observe fungal growth associated with plant sections and record the occurrence of B. bassiana to estimate the extent of its endophytic colonization. Analyses of inoculation success compare the occurrence of B. bassiana within a given plant part (i.e. leaves, stems or roots) across treatments and controls. In addition to the inoculation method, the specific outcome of the experiment may depend on the target crop species or variety, the fungal entomopathogen species strain or isolate used, and the plant's growing conditions.
Introduction
Fungal entomopathogens are important regulators of insect populations with considerable potential as mycopesticides1. Only recently, however, have fungal entomopathogens been shown to occur as endophytes, both naturally and in response to various inoculation methods2. The ecological function of endophytic fungal entomopathogens remains largely unknown, but some studies have implicated them in plant growth3,4, herbivore resistance5-8, and disease resistance9,10. The overall objective of the methods presented here is to introduce a fungal entomopathogen as an endophyte, in preparation for subsequent evaluations of endophytic biological control.
Beauveria bassiana (Balsamo) Vullemin (Ascomycota: Hypocreales) is the best-studied endophytic fungal entomopathogen5-9,11-19, and it is available as a commercial mycopesticide. Inoculation methods tested to establish B. bassiana as an endophyte include soil drenches14,17, seed coatings18 and immersions14, radicle dressings13,15, root and rhizome immersions11,16,18, stem injections17, foliar sprays14,17,20 and flower sprays19. Using these methods, researchers have introduced B. bassiana into banana11, bean7, cacao13, coffee17, corn7, cotton7, date palm12, jute21, opium poppy20, pumpkin7, radiata pine18, sorghum14, tomato7 and wheat7. Recent evidence suggests that endophytic B. bassiana has the potential to protect plants not only from arthropod pests5-7,22-27, but also from some plant pathogens9.
The common bean (Phaseolus vulgaris) ranks among the crops most vulnerable to pests and diseases. It can be affected by more than 400 pests and 200 pathogens, whose attack is thought to be the most limiting bean production factor across regions28. Accordingly, the common bean may be an excellent model crop to examine the full spectrum of endophytic biological control by B. bassiana. As a first step in this direction, this article describes foliar sprays and soil drenches as inoculation methods to introduce B.bassiana as an endophyte in the common bean.
Protocol
1. Plants
- Surface-sterilize bean seeds (cv. Calima) by immersing them for two minutes in 0.5% sodium hypochlorite and two minutes in 70% ethanol. Rinse the seeds three times in sterile distilled water.
- Evaluate the success of your sterilization by plating 100 μl of the last rinsing water on potato dextroxe agar (PDA) media, and incubating the plate for 10 days at 25 °C. Terminate and restart the experiment if any growth is seen on the plate.
- Plant the seeds in groups of three in pots containing a sterile mixture of soil and sand at a 2:1 ratio. Transfer the pots to a growth chamber at 25 °C, ca. 50% RH and 12 hr photoperiod. One week after germination, eliminate the two least vigorous seedlings. Water every 2-3 days with sterile distilled water and fertilize 10 and 20 days after planting with a 6 g/L water solution of NPK 15-15-15 fertilizer.
2. Fungus
- Obtain a commercial formulation of Beauveria bassiana strain GHA (Mycotrol SE, Laverlam, Cali, Colombia).
- To generate a single-spore stock culture, suspend ca. one inoculating loop full of conidia in 1 ml of a 0.1% water solution of Triton-X 100 and vortex for 10 sec. Then, plate 100 μl of the suspension on 2.5% Noble agar and incubate for 24 hr at 25 °C. Transfer a single germinating conidium into a 100 mm plate containing PDA and grow until it covers the entire plate (ca. 3-4 weeks).
- Under sterile conditions, scrape the fungal growth from the surface of the medium and suspend it in 10 ml sterile 0.1% Triton-X 100. Vortex for one minute. Then, filter the suspension through a sterile cheesecloth to remove hyphae and obtain the stock suspension.
- Use a hemocytometer to estimate the conidial concentration of the stock suspension. To facilitate conidial counts, prepare a 10,000-fold serial dilution of the stock, each time transferring 100 μl of conidial suspension in 900 μl of 0.1% Triton-X 100, and vortexing for 10 sec before the next dilution.
- To generate the inoculum, adjust the stock suspension to a final concentration of 108 conidia/ml, using the formula:
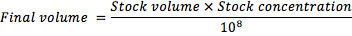
- To assess conidial viability, plate 100 μl of the 10,000-fold dilution on 2.5% Noble agar and incubate for 24 hr at 25 °C. Then, inspect three random groups of 100 conidia to estimate percent germination. Consider a conidium germinated when a visible germ tube longer than half the diameter of the conidium projects from it. Only use the suspension when the average percent germination exceeds 90%.
3. Inoculation
- Inoculate plants when they reach their first true leaf stage (ca. 14 days after planting). Water plants to soil capacity with sterile distilled water 24 hr before inoculations.
- For the foliar spray method, use a manual atomizer to apply the conidial suspension (treatment) or 0.1% Triton-X 100 (control) to the adaxial (upper) surface of leaves until they reach saturation. Cover the top of the pot with aluminum foil to avoid conidial runoff to the soil. After spraying, cover plants with a plastic bag for 24 hr to maintain a high level of humidity facilitating fungal invasion.
- For the soil drench method, use a graduated cylinder to apply 10 ml of conidial suspension (treatment) or 0.1% Triton-X 100 (control) to the surface of the soil at the base of the plant.
- After inoculations, return plants to the growth chambers arranging them in a randomized complete block design. No less than four additional experimental blocks should be installed to allow evaluations of plant growth, in addition to evaluations of endophytic colonization, in the same experiment.
4. Evaluations
- Evaluate the experiment one block at a time, selecting the blocks in random order. This is particularly important for large experiments that cannot be evaluated on a single day.
- Before processing a plant, measure and record its height from the base to the apical meristem. Then, uproot carefully and wash thoroughly in running tap water.
- From each plant, sample two leaflets, two pieces of root and two pieces of stem. Select leaflet samples randomly from the first true leaf of the plant. Then, obtain two stem samples, 3 cm-long each, from the middle of the plant and from near the soil surface. Finally, obtain two taproot samples, also 3 cm-long each, from the middle of the root and from 1 cm behind the root tip. Place the samples on three separate paper bags and label appropriately.
- After washing and sampling all the plants in a block, start by processing the leaves, then roots, and finally the stems.
- Surface sterilize tissues in a sterile laminar flow hood as in 1.1, above. Rinse each sample three times by immersion in sterile distilled water and let it dry in sterile towel paper. Then, dissect and discard its outer edges, where endophytes might have been eliminated due to contact with disinfectants.
- Cut the trimmed sample into six sections, averaging 6x6 mm for leaves and 6 mm-long for stems and roots. Plate the six sections on a 60 mm Petri plate with PDA media supplemented with the antibiotics tetracycline, streptomycin and penicillin at 2 mg/L each. Seal the plate with parafilm and incubate in the dark at 25 °C. Each plant yields six plates, two per plant part.
- Change the rinse water after processing each block of a given plant part. Before discarding the used rinsing water, plate a 100 μl sample on PDA media and incubate for 10 days at 25 °C to assess sterilization success. If fungal growth ensues, do not consider the corresponding samples for analyses.
- Inspect the plates every 2-3 days for 20 days to observe and record fungal growth. Excise and transfer plant sections exhibiting presence of fungal endophytes to plates containing fresh PDA. This will avoid contamination of neighboring plant sections in the original plate.
- Record B. bassiana growth from plant sections. Beauveria bassiana can be identified by characteristic white dense mycelia becoming cream to pale yellow at the edge. When in doubt, mount the specimen in a drop of water and inspect under a microscope, looking for globose conidia and zigzag-shaped conidiophores, characteristic of the species.
- Use additional experimental blocks to evaluate the impact of the treatments on plant biomass. First, measure their height from the base to the top of the apical meristem. Then, carefully uproot and wash plants in tap water and let them dry at 45 °C for three days to determine their dry weight.
Results
B. bassiana was able to endophytically-colonize P. vulgaris in response to the demonstrated inoculation treatments (Figure 1). Both foliar sprays and soil drenches resulted in endophytic colonization by B. bassiana in over 80% of the treated plants (Figure 2). However, the extent of colonization depended on the plant part evaluated and the inoculation method used. Leaves responded best to spray inoculations. Roots, on the other hand, responded only to drench inoculations. Finally, stems responded similarly to both inoculation methods. B. bassiana was not detected in any of the control plant sections.
Independent of the treatment, endophytes other than B. bassiana grew from 15% of the evaluated plant sections, but they were dissected out from media plates before they could invade neighboring sections and influence the results.
Treatment and control plants were visibly indistinguishable two weeks after inoculations. No differences were detected in their dry weight and in their height.
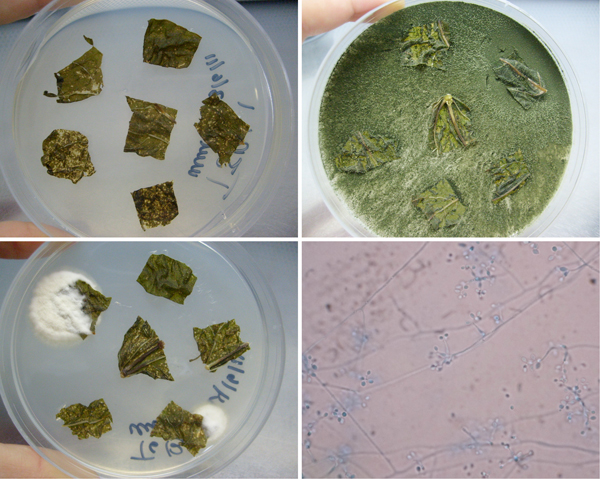
Figure 1. Representative results of inoculation treatments on endophytic colonization of bean plants (Phaseolus vulgaris cv. Calima) by Beauveria bassiana. Top left: control plates with no growth. Top right: Fungal endophyte from a plant section contaminating entire plate. Bottom left: B. bassiana growing from two plant sections. Bottom right: Endophytic B. bassiana conidia and conidiophores as seen under a microscope.
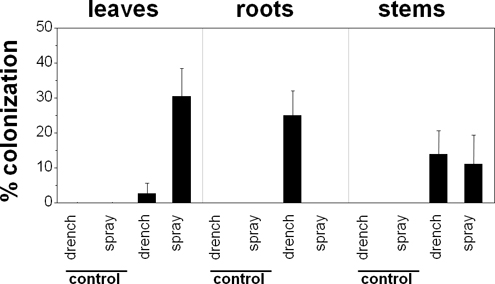
Figure 2. Effect of inoculation treatments on endophytic colonization of bean plants (Phaseolus vulgaris cv. Calima) by Beauveria bassiana, two weeks after inoculations with the strain GHA. Percent colonization represents the number of colonized plant sections divided by the number of cultured sections.
Discussion
Many factors can influence the specific outcome of an experiment to establish a fungal entomopathogen as an endophyte. Our results demonstrate the inoculation method is one of them. Biological factors to experiment with include the crop species or cultivar selected and the fungal entomopathogen species strain or isolate used. Other factors to consider manipulating include the concentration of the inoculum, the age of the plant during inoculations, and the plant's growing conditions.
It would be ideal for an inoculation method to result in systemic plant colonization by a fungal entomopathogen14,17,18,21. Instead, it appears that inoculation methods tend to favor a specific pattern of local colonization. In coffee, for example, foliar sprays favor leaf colonization whereas soil drenches favor root colonization17. We found the same pattern in the common bean. Ultimately, the choice of inoculation method should be guided by the intended location of the endophyte within a plant, presumably matching the niche of the target herbivore or plant pathogen.
Although commonly used, endophyte detection and quantification based on media cultures can be costly, difficult and error prone. For example, a total of 10,800 plant sections (plated on 1,800 Petri dishes) were evaluated in an experiment to optimize B. bassiana inoculations on banana16. Out of these, 4,496 sections were colonized by a putative B. bassiana, as identified mainly by colony morphology. Clearly, a microscopic verification of the species for each colony would have been a desirable but unaffordable step. On the other hand, 1,176 sections were colonized by other fungi and were discarded and treated as missing data16. The probability exists, however, that B. bassiana was a poor competitor or slower to grow, and could have eventually emerged from those sections if allowed sufficient time. Therefore, endophyte detection methods based on media cultures are subject to false positives and false negatives. Accordingly, the development of more reliable detection and quantification methods, for example those based on polymerase chain reaction (PCR) assays20,21,24,is well justified.
The ultimate goal for inoculation experiments should be to develop an efficient treatment that provides durable systemic resistance against herbivory and/or disease. A plausible, but as yet untested, hypothesis is that the extent of endophytic colonization should correlate positively with the extent of endophyte-mediated resistance. A natural next step after refining inoculation methods, therefore, could be to examine this correlation. Several video protocols can help researchers design a suitable resistance assay for a target pest or pathogen29-31. Ultimately, it is this assay what will determine the success of the inoculation method, and the associated potential for endophytic biological control.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
The production and the experimental work presented herein reflect the devoted and enthusiastic help of Reynaldo Pareja. Funded by Colombia's Administrative Department of Science, Technology and Innovation (Colciencias) and by a grant from the Bill & Melinda Gates Foundation through the Grand Challenges Explorations initiative.
Materials
| Name | Company | Catalog Number | Comments |
| REAGENTS | |||
| Name of Reagent/Material | Company | Catalog Number | |
| Mycotrol SE | Laverlam | 4167 | |
| Noble agar | Sigma | A5431-250G | |
| Penicillin G sodium salt | Sigma | P3032-25MU | |
| Petri dish (100 x 15 mm) | Fisher | 08-757-12 | |
| Petri dish (60 x 15 mm) | Fisher | 08-757-13A | |
| Potato dextrose agar | Difco | 213400 | |
| Regular bleach (NaOCl) | CLOROX | N/A | |
| Streptomycin sulfate salt | Sigma | S6501-25G | |
| Tetracycline | Sigma | T3258-25G | |
| Triple quince (NPK) | ABOCOL | N/A | |
| Triton X-100 | Sigma | X-100 | |
| EQUIPMENT | |||
| Biological safety cabinet | NuAire | NU-425-600 | |
| Hemocytometer | Fisher | 02-671-10 | |
| Leica DM LB microscope | Leica | N/A | |
References
- Vega, F. E., Meyling, N. V., Luangsa-ard, J. J., Blackwell, M., Vega, F. E., Kaya, H. K. . Fungal entomopathogens in Insect Pathology. , 171-220 (2012).
- Vega, F. E. Insect pathology and fungal endophytes. Journal of invertebrate pathology. 98, 277-279 (2008).
- Sasan, R. K., Bidochka, M. J. The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. American journal of botany. 99, 101-107 (2012).
- Elena, G. J., Beatriz, P. J., Alejandro, P. Metarhizium anisopliae (Metschnikoff) Sorokin promotes growth and has endophytic activity in tomato plants. Advances in biological research. 5, 22-27 (2011).
- Bing, L. A., Lewis, L. C. Suppression of Ostrinia nubilalis (Hübner)(Lepidoptera: Pyralidae) by endophytic Beauveria bassiana (Balsamo) Vuillemin. Environmental entomology. 20, 1207-1211 (1991).
- Akello, J., Dubois, T., Coyne, D., Kyamanywa, S. Endophytic Beauveria bassiana in banana (Musa spp.) reduces banana weevil (Cosmopolites sordidus) fitness and damage. Crop protection. 27, 1437-1441 (2008).
- Gurulingappa, P., Sword, G. A., Murdoch, G., McGee, P. A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biological. 55, 34-41 (2010).
- Akello, J., Dubois, T., Coyne, D., Kyamanywa, S. Effect of endophytic Beauveria bassiana on populations of the banana weevil, Cosmopolites sordidus, and their damage in tissue-cultured banana plants. Entomologia experimentalis et applicata. 129, 157-165 (2008).
- Ownley, B. H., et al. Beauveria bassiana: Endophytic colonization and plant disease control. Journal of invertebrate pathology. 98, 267-270 (2008).
- Ownley, B. H., Gwinn, K. D., Vega, F. E. Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. BioControl. 55, 113-128 (2010).
- Akello, J., et al. Beauveria bassiana (Balsamo) Vuillemin as an endophyte in tissue culture banana (Musa spp.). Journal of invertebrate pathology. 96, 34-42 (2007).
- Gómez-Vidal, S., Lopez-Llorca, L. V., Jansson, H. B., Salinas, J. Endophytic colonization of date palm (Phoenix dactylifera L.) leaves by entomopathogenic fungi. Micron. 37, 624-632 (2006).
- Posada, F., Vega, F. E. Establishment of the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte in cocoa seedlings (Theobroma cacao). Mycologia. 97, 1195-1200 (2005).
- Tefera, T., Vidal, S. Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. BioControl. 54, 663-669 (2009).
- Posada, F., Vega, F. E. Inoculation and colonization of coffee seedlings (Coffea arabica L.) with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales. Mycoscience. 47, 284-289 (2006).
- Akello, J., Dubois, T., Coyne, D., Kyamanywa, S. The effects of Beauveria bassiana dose and exposure duration on colonization and growth of tissue cultured banana (Musa sp.) plants. Biological. 49, 6-10 (2009).
- Posada, F., Aime, M. C., Peterson, S. W., Rehner, S. A., Vega, F. E. Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycological research. 111, 748-757 (2007).
- Brownbridge, M., Reay, S. D., Nelson, T. L., Glare, T. R. Persistence of Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte following inoculation of radiata pine seed and seedlings. Biological control. 61, 194-200 (2012).
- Posada, F. J., Chaves, F. C., Gianfagna, T. J., Pava-Ripoll, M., Hebbar, P. Establishment of the fungal entomopathogen Beauveria bassiana as an endophyte in cocoa pods (Theobroma cacao L.). Revista U.D.C.A. actualidad & divulgación científica. 13, 71-78 (2010).
- Quesada-Moraga, E., Landa, B. B., Muñoz-Ledesma, J., Jiménez-Díaz, R. M., Santiago-Alvarez, C. Endophytic colonisation of opium poppy, Papaver somniferum, by an entomopathogenic Beauveria bassiana strain. Mycopathologia. 161, 323-329 (2006).
- Biswas, C., Dey, P., Satpathy, S., Satya, P. Establishment of the fungal entomopathogen Beauveria bassiana as a season long endophyte in jute (Corchorus olitorius) and its rapid detection using SCAR marker. BioControl. , 1-7 (2011).
- Bing, L. A., Lewis, L. C. Occurrence of the entomopathogen Beauveria bassiana (Balsamo) Vuillemin in different tillage regimes and in Zea mays L. and virulence towards Ostrinia nubilalis (Hübner). Agriculture, ecosystems & environment. 45, 147-156 (1993).
- Akello, J., Sikora, R. Systemic acropedal influence of endophyte seed treatment on Acyrthosiphon pisum and Aphis fabae offspring development and reproductive fitness. Biological. 61, 215-221 (2012).
- Reddy, N. P., Ali Khan, A. P., Devi, U. K., Sharma, H. C., Reineke, A. Treatment of millet crop plant (Sorghum bicolor) with the entomopathogenic fungus (Beauveria bassiana) to combat infestation by the stem borer, Chilo partellus Swinhoe (Lepidoptera: Pyralidae. Journal of Asia Pacific. 12, 221 (2009).
- Quesada-Moraga, E., Muñoz-Ledesma, F. J., Santiago-Alvarez, C. Systemic protection of Papaver somniferum L. against Iraella luteipes (Hymenoptera: Cynipidae) by an endophytic strain of Beauveria bassiana (Ascomycota: Hypocreales). Environmental entomology. 38, 723-730 (2009).
- Cherry, A. J., Banito, A., Djegui, D., Lomer, C. Suppression of the stem-borer Sesamia calamistis (Lepidoptera; Noctuidae) in maize following seed dressing, topical application and stem injection with African isolates of Beauveria bassiana. International journal of pest management. 50, 67-73 (2004).
- Gurulingappa, P., McGee, P. A., Sword, G. Endophytic Lecanicillium lecanii and Beauveria bassiana reduce the survival and fecundity of Aphis gossypii following contact with conidia and secondary metabolites. Crop protection. 30, 349-353 (2011).
- van Schoonhoven, A., Voysest, O., Schwartz, H. F., Pastor-Corrales, M. A. . Common beans in Latin America and their constraints in Bean production problems in the tropics. , 33-57 (1989).
- De Vos, M., Jander, G. Choice and no-choice assays for testing the resistance of A. thaliana to chewing insects. J. Vis. Exp. (15), e683 (2008).
- Parsa, S., Sotelo, G., Cardona, C. Characterizing herbivore resistance mechanisms: spittlebugs on Brachiaria spp. as an example. J. Vis. Exp. (52), e3047 (2011).
- Atamian, H., Roberts, P., Kaloshian, I. High and low throughput screens with root-knot nematodes Meloidogyne spp. J. Vis. Exp. (61), e3629 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved