A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Photopatterning Proteins and Cells in Aqueous Environment Using TiO2 Photocatalysis
In This Article
Summary
We describe a protocol for modifying cell affinity of a scaffold surface in aqueous environment. The method takes advantage of titanium dioxide photocatalysis to decompose organic film in the photo-irradiated region. We show that it can be used to create microdomains of scaffolding proteins, both ex situ and in situ.
Abstract
Organic contaminants adsorbed on the surface of titanium dioxide (TiO2) can be decomposed by photocatalysis under ultraviolet (UV) light. Here we describe a novel protocol employing the TiO2 photocatalysis to locally alter cell affinity of the substrate surface. For this experiment, a thin TiO2 film was sputter-coated on a glass coverslip, and the TiO2 surface was subsequently modified with an organosilane monolayer derived from octadecyltrichlorosilane (OTS), which inhibits cell adhesion. The sample was immersed in a cell culture medium, and focused UV light was irradiated to an octagonal region. When a neuronal cell line PC12 cells were plated on the sample, cells adhered only on the UV-irradiated area. We further show that this surface modification can also be performed in situ, i.e., even when cells are growing on the substrate. Proper modification of the surface required an extracellular matrix protein collagen to be present in the medium at the time of UV irradiation. The technique presented here can potentially be employed in patterning multiple cell types for constructing coculture systems or to arbitrarily manipulate cells under culture.
Introduction
Semiconductor lithography processes and its derivatives — such as photolithography1,2, electron-beam lithography3-6, and microcontact printing7-10 — have now become an established tool in cell biology to grow living cells in a defined position and geometry. The patterning method relies on the use of microfabricated substrates, consisting of micro-island of cell permissive coating in a non-permissive background. Such substrate serves as a template to pattern the cells. These technologies have provided us the novel methods to engineer cells and their function at a single- and multi-cellular level, to extract the intrinsic properties of cells, and to increase the throughput of cell-based drug screening11.
The degree-of-freedom in cell patterning would greatly increase if the template pattern geometry could be altered in situ, i.e., while cells are cultured on the surface. The conventional methods for pattern formation cannot be directly applied here, since they process samples in atmosphere or in vacuum. Therefore various new surface modification techniques have been proposed, which are based, e.g., on photoreactive compounds12,13 or laser ablation5,14, just to name a few. The proposed methods have been nicely reviewed by Robertus et al.15, and more recently by Choi et al.16 and by Nakanishi17.
Here in this article, we describe a novel protocol of in-situ surface modification, which takes advantage of photocatalytic decomposition of organic molecules on a titanium dioxide (TiO2) surface18,19. In this method, a TiO2 film is inserted between the glass substrate and the organic film that interfaces the cells, and the organic film is decomposed in situ by locally irradiating ultraviolet (UV) light to a region of interest (λ < 388 nm). We show that the new protocol can be used to create micropatterns of extracellular matrix proteins and living cells both ex situ and in situ. TiO2 is biocompatible, chemically stable, and optically transparent, features of which makes it friendly to introduce in cell-culture experiments. This protocol provides a materials science-based alternative for modifying cell-culture scaffolds in cell-culture environment.
Protocol
1. Preparation of TiO2-coated Glass Coverslip
- Number the coverslips using a diamond scriber. This helps not only to keep track of each coverslips but also to ensure that the correct side of the sample is facing up. Clean the coverslips, first under running ddH2O, then by immersing them in piranha solution (H2SO4:H2O2 = 4:1). After 10 min, rinse the coverslips thoroughly, 8 times in ddH2O. Dry the coverslips under N2 flow.
- Set TiO2 target in the radio-frequency (RF) sputtering system. Attach the coverslips onto a sample holder of the sputtering equipment using a polyimide tape. Place the sample holder in the sputtering chamber. Evacuate the chamber until the pressure reaches 2.0×10-4 Pa.
- Introduce Ar gas into the chamber and set the deposition pressure to 4.0 mTorr. While keeping the shutter closed, gradually increase the RF power to 70 W.
- Open the shutter and sputter for 15 min to obtain a film with a thickness of 120-150 nm (Figure 1: Step A). Growth rate of the film needs to be derived for each machine.
2. Surface Coating with Cell-repellent Film
- Hydrophilize the TiO2 surface by treating the sample with O2 plasma, following instructions provided by the manufacturer of the plasma reactor. We treat the sample for 5 min at 200 W with O2 flow of 100 sccm. Immerse the sample in ddH2O and confirm that the surface is superhydrophilic. Dry the surface thoroughly under N2 flow.
- Prepare 1 mM octadecyltrichlorosilane (OTS) solution by adding 39.6 μl OTS to 100 ml toluene. Immerse the sample in the solution for 1 hr at RT. Conduct this step inside an N2-filled glove bag (Figure 1: Step B).
- To remove physisorbed molecules, sonicate the sample in toluene, acetone, ethanol, and ddH2O for 5 min each, in that order. Rinse the sample four times in fresh ddH2O and dry the surface under N2 flow. The surface should be hydrophobic with a contact angle of 100-110°.
3. Ex-Situ Surface Patterning
- Working in a laminar flow hood, draw several scratch marks with a diamond scriber on the surface. The marks help in keeping track of the processed regions and also bringing microscopes into focus. Sterilize the OTS-coated TiO2 by immersing the sample in 70% ethanol for 5 min. Then rinse the sample twice in sterilized ddH2O.
- Place the sample in a 35-mm dish, and add 2 ml of PC12 growth medium (see Step 4.2). Incubate for over 3 hr in a CO2 incubator (37 °C). This procedure is intended to let serum albumins absorb onto the surface. Adsorbed albumins inhibit subsequent adsorption of other proteins and cells (Figure 1: Step C).
- While waiting, set up the inverted fluorescence microscope.
- Turn on the arc lamp, insert the UV filter cube, and set the objective lens to 20X.
- Measure light intensity I (W cm-2) using an UV-intensity meter, and calculate irradiation time t (in seconds) for a dose of d (in J cm-2) as: t = d/I.
For instance, to irradiate at a dose of 200 J cm-2 using a light source of 600 mW cm-2, 333 sec of irradiation is required. - Use a stage micrometer and close the field diaphragm to set the size of the region to be irradiated, e.g., 200 μm.
- After the 3 hr incubation, supplement the medium with 200 μl of 3.0 mg ml-1 type-IV collagen (Col-IV; final concentration 300 μg ml-1).
- Transfer the 35-mm dish to the microscope stage. Find the scratch mark, focus the microscope onto the sample surface, and irradiate UV-light at a dose of 200 J cm-2 (Figure 1: Step D). The area of UV irradiation can be altered either by adjusting the opening of the field diaphragm or by replacing the field diaphragm with a metal mask of arbitral geometry.
- Replace the medium with a fresh growth medium (without Col-IV), and place the sample back in the incubator.
4. Cell Culture
- Routine culture of PC12 cells is carried out on a collagen-coated plastic dish.
- To coat a 60-mm tissue-culture dish with Col-IV, first wet the surface with ddH2O and aspirate all ddH2O.
- Prepare 300 μg ml-1 Col-IV by diluting the original solution (3 mg ml-1) 10x with ddH2O.
- Add 200 μl of 300 μg ml-1 Col-IV per 60-mm dish. Let the solution spread through the surface.
- Dry the dish in a laminar flow hood for approximately 1 hr.
- Rinse the surface twice with 4 ml of Dulbecco's phosphate-buffered saline (D-PBS). When not using the coated dish immediately, rinse the dish once with 4 ml of ddH2O. After aspirating ddH2O, store the dish in the incubator. Try not to keep it stored for more than two weeks.
- PC12 cells are grown in a growth medium that consists of: Dulbecco's modified Eagle's medium (low glucose) + 10% fetal bovine serum + 5% horse serum + 1% penicillin-streptomycin solution. Incubate the cells in a CO2 incubator (37 °C, 5% CO2), and replace half of the medium every other day. Passage cells before they reach confluence.
- Aspirate the growth medium, and add 2 ml of PBS+ (D-PBS + 10 mg ml-1 bovine serum albumin (BSA) + 10 mM EDTA) prewarmed to 37 °C. Incubate for 5 min at 37 °C. Gently tap the dish to detach all cells.
- Collect cells in a 15 ml conical tube. Rinse the dish with 3 ml of fresh D-PBS, prewarmed to 37 °C.
- Centrifuge the tube at 150 × g for 4 min.
- Aspirate the supernatant, and add 1 ml of the growth medium.
- For routine culture, split the cells 1:3 to 1:5.
- To plate cells on the UV-modified OTS/TiO2 sample, count cell density and add 3.0×105 cells in a 35-mm dish (Figure 1: Step E). Incubate the dish in the humidified incubator (37 °C, 5% CO2) for 1-2 days. Both naive and nerve growth factor (NGF)-differentiated PC12 cells can be used for the patterning experiments. For NGF differentiation, 100 ng ml-1 of 7S-NGF is added to the growth medium several days before plating the cells onto the sample. NGF-differentiation seems to increase the adhesibility of the PC12 cells and makes handling easier, especially in the in-situ patterning experiments.
5. In-Situ Surface Patterning
- After 1-2 days of culture on the ex-situ modified surface, confirm that the cells are attaching and growing only on the UV-irradiated region. Set up the microscope, as described in Step 3.4.
- Transfer the sample to a new 35-mm tissue culture dish containing the growth medium, 100 ng ml-1 NGF (in the case of using NGF-differentiated cells), and 100 μg ml-1 Col-IV.
- Place the dish on the microscope stage. Find the appropriate position, and irradiate UV light at a dose of 200 J cm-2 (Figure 1: Step F, G). The newly created permissive region is indicated with an asterisk in Figure 1: Step G.
- Try to complete processing of a single sample within 30 min. After irradiation, transfer the sample back into the medium without Col-IV.
- Be extremely careful when carrying the dish with cultured cells and transferring the sample from dish to dish to prevent detaching of the patterned cells.
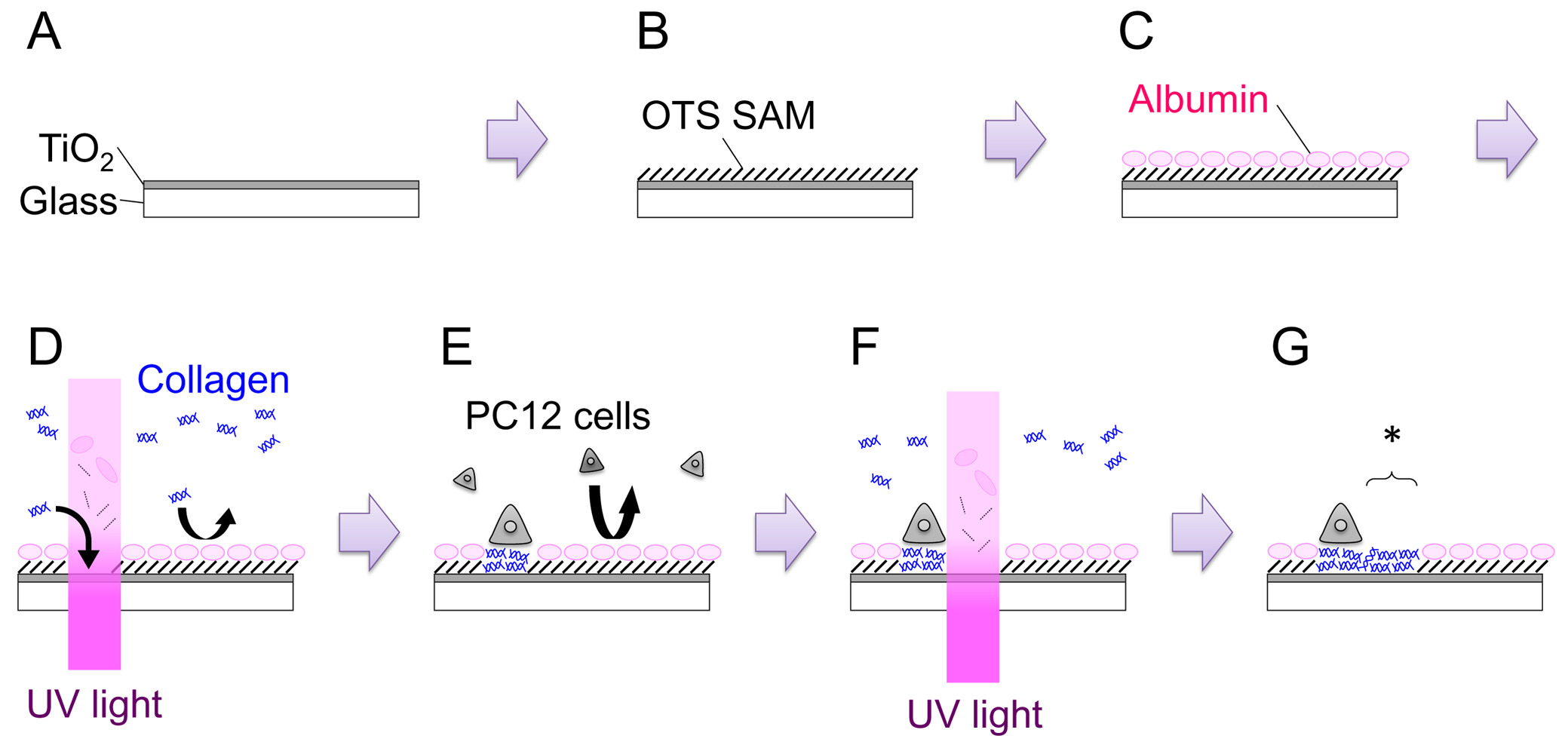
Figure 1. Schematic illustration of the overall process. See text for details. Please click here to view a larger version of this figure.
Results
Figure 2A shows a cross-sectional scanning electron microscopy (SEM) image of the sputter-deposited TiO2 film. From the observation, thickness of the film was estimated to be approximately 150 nm. Noticeable here is the flatness of the deposited TiO2 film. Further analysis by atomic force microscopy (AFM) revealed that the root-mean-square (rms) roughness of the surface was 0.2 nm (Figure 2B).
When the TiO2 surface is modified ...
Discussion
In our current protocol, TiO2 film was formed by RF-magnetron sputtering. We favor this method of deposition since it allows us to reproducibly prepare a photocatalytic TiO2 film with a sub-nm roughness. Although sputter deposition processes are familiar to materials scientists and electronic engineers, it may not be quite accessible to biologists. In that case, spin-coated TiO2 film would be an alternative choice23. In this method, TiO2 nanoparticles dissolved in a ...
Disclosures
The authors have nothing to disclose.
Acknowledgements
Authors thank Mr. Kotaro Okubo for the kind assistance with SEM imaging. This work was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Basic Research (B) (20310069), Grant-in-Aid for Research Activity Start-up (25880021), and by research grants from the Kurata Memorial Hitachi Science and Technology Foundation and the Nippon Sheet Glass Foundation for Materials Science and Engineering.
Materials
| Name | Company | Catalog Number | Comments |
| Glass coverslip | Warner Instruments | CS-15R15 | 15 mm diameter, #1.5 thickness |
| Diamond scriber | Ogura Jewel Industry | D-Point Pen | |
| RF sputtering system | ANELVA | SPC350 | |
| TiO2 sputtering target | Kojundo Chemical Lab | Titanium (IV) oxide, target | Purity, 99.9% |
| Plasma reactor | Yamato | PR301 | |
| n-octadecyltrichlorosilane (OTS) | Aldrich | 104817 | |
| Toluene | Wako | 204-01866 | |
| Tissue-culture dish (35 mm) | Greiner | 627160 | |
| Tissue-culture dish (60 mm) | BD Falcon | 353002 | |
| Type-IV collagen | Nitta Gelatin | Cellmatrix Type IV | |
| D-PBS | Gibco | 14190-144 | |
| Dulbecco's modified Eagle's medium (DMEM) | Gibco | 11885-084 | |
| Fetal bovine serum | Gibco | 12483-020 | Heat-inactivate and pass through a 0.22 μm filter before use |
| Horse serum | Gibco | 26050-088 | Pass through a 0.22 μm filter before use |
| Penicillin-streptomycin (100x) | Nacalai tesque | 26253-84 | |
| 7S nerve growth factor (NGF) | Alomone Labs | N-130 | |
| Bovine serum albumin (BSA) | Sigma | A2153 | |
| EDTA | Dojindo | N001 | Stock solution in 0.5 M |
| TiO2 nanoparticle | Tayca | TKD-701 |
References
- Hughes, M. A., Brennan, P. M., Bunting, A. S., Shipston, M. J., Murray, A. F. Cell Patterning on Photolithographically Defined Parylene-C: SiO2 Substrates. J. Vis. Exp. (85), e50929 (2014).
- Kleinfeld, D., Kahler, K. H., Hockberger, P. E. Controlled Outgrowth of Dissociated Neurons on Patterned Substrates. J. Neurosci. 8, 4098-4120 (1988).
- Pensen, D., Heinz, W. F., Werbin, J. L., Hoh, J. H., Haviland, D. B. Electron Beam Patterning of Fibronectin Nanodots that Support Focal Adhesion Formation. Soft Matter. 3, 1280-1284 (2007).
- Tanii, T., et al. Application of Organosilane Monolayer Template to Quantitative Evaluation of Cancer Cell Adhesive Ability. Jpn. J. Appl. Phys. 50, 06GL01 (2011).
- Yamamoto, H., et al. In-Situ Guidance of Individual Neuronal Processes by Wet Femtosecond Laser Processing of Self-Assembled Monolayers. Appl. Phys. Lett. 99, 163701-1610 (2011).
- Yamamoto, H., et al. Differential Neurite Outgrowth is Required for Axon Specification by Cultured Hippocampal Neurons. J. Neurochem. 123, 904-910 (2012).
- Shen, K., Qi, J., Kam, L. C. Microcontact Printing of Proteins for Cell Biology. J. Vis. Exp. (22), e1065 (2008).
- Johnson, D. M., LaFranzo, N. A., Maurer, J. A. Creating Two-Dimensional Patterned Substrates for Protein and Cell Confinement. J. Vis. Exp. (55), e3164 (2011).
- Singhvi, R., et al. Engineering Cell Shape and Function. Science. 264, 696-698 (1126).
- Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M., Ingber, D. E. Geometric Control of Cell Life and Death. Science. 276, 1425-1428 (1997).
- Degot, S., et al. Improved Visualization and Quantitative Analysis of Drug Effects using Micropatterned Cells. J. Vis. Exp. (46), e2514 (2010).
- Nakanishi, J., et al. Photoactivation of a Substrate for Cell Adhesion under Standard Fluorescence Microscopes. J. Am. Chem. Soc. 126, 16314-16315 (2004).
- Kim, M., et al. Addressable Micropatterning of Multiple Proteins and Cells by Microscope Projection Photolithography Based on a Protein Friendly Photoresist. Langmuir. 26, 12112-12118 (2010).
- Deka, G., Okano, K., Kao, F. -. J. Dynamic Photopatterning of Cells In Situ by Q-Switched Neodymium-Doped Yttrium Ortho-Vanadate. Laser. J. Biomed. Opt. 19, 011012 (2014).
- Robertus, J., Browne, W. R., Feringa, B. L. Dynamic Control over Cell Adhesive Properties using Molecular-Based Surface Engineering Strategies. Chem. Soc. Rev. 39, 354-378 (2010).
- Choi, I., Yeo, W. -. S. Self-Assembled Monolayers with Dynamicity Stemming from (Bio)chemical Conversions: From Construction to Application. ChemPhysChem. 14, 55-69 (2013).
- Nakanishi, J. Switchable Substrates for Analyzing and Engineering Cellular Functions. Chem. Asian J. 9, 406-417 (2014).
- Yamamoto, H., et al. In Situ Modification of Cell-Culture Scaffolds by Photocatalytic Decomposition of Organosilane Monolayers. Biofabrication. 6, 035021 (2014).
- Sekine, K., Yamamoto, H., Kono, S., Ikeda, T., Kuroda, A., Tanii, T. Surface Modification of Cell Scaffold in Aqueous Solution using TiO2 Photocatalysis and Linker Protein L2 for Patterning Primary Neurons. e-J. Surf. Sci. Nanotech. 13, 213-218 (2015).
- Arima, Y., Iwata, H. Effects of Surface Functional Groups on Protein Adsorption and Subsequent Cell Adhesion using Self-Assembled Monolayers. J. Mater. Chem. 17, 4079-4087 (2007).
- Fujishima, A., Zhang, X., Tryk, D. A. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 63, 515-582 (2008).
- Sigal, G. B., Mrksich, M., Whitesides, G. M. Effect of Surface Wettability on the Adsorption of Proteins and Detergents. J. Am. Chem. Soc. 120, 3464-3473 (1998).
- Zhang, X., et al. A Transparent and Photo-Patternable Superhydrophobic Film. Chem. Commun. 2007, 4949-4951 (1039).
- Kaech, S., Banker, G. Culturing Hippocampal Neurons. Nat. Protoc. 1, 2406-2415 (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved