A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Fabricating a UV-Vis and Raman Spectroscopy Immunoassay Platform
* These authors contributed equally
In This Article
Summary
Nanoparticle-based optical probes have been designed as a vehicle for detecting antigens using Raman and UV-Vis spectroscopy. Here we describe a protocol for preparing such probes for a UV-Vis/Raman spectroscopy immunoassay in such a way to incorporate future multiplexing capabilities.
Abstract
Immunoassays are used to detect proteins based on the presence of associated antibodies. Because of their extensive use in research and clinical settings, a large infrastructure of immunoassay instruments and materials can be found. For example, 96- and 384-well polystyrene plates are available commercially and have a standard design to accommodate ultraviolet-visible (UV-Vis) spectroscopy machines from various manufacturers. In addition, a wide variety of immunoglobulins, detection tags, and blocking agents for customized immunoassay designs such as enzyme-linked immunosorbent assays (ELISA) are available.
Despite the existing infrastructure, standard ELISA kits do not meet all research needs, requiring individualized immunoassay development, which can be expensive and time-consuming. For example, ELISA kits have low multiplexing (detection of more than one analyte at a time) capabilities as they usually depend on fluorescence or colorimetric methods for detection. Colorimetric and fluorescent-based analyses have limited multiplexing capabilities due to broad spectral peaks. In contrast, Raman spectroscopy-based methods have a much greater capability for multiplexing due to narrow emission peaks. Another advantage of Raman spectroscopy is that Raman reporters experience significantly less photobleaching than fluorescent tags1. Despite the advantages that Raman reporters have over fluorescent and colorimetric tags, protocols to fabricate Raman-based immunoassays are limited. The purpose of this paper is to provide a protocol to prepare functionalized probes to use in conjunction with polystyrene plates for direct detection of analytes by UV-Vis analysis and Raman spectroscopy. This protocol will allow researchers to take a do-it-yourself approach for future multi-analyte detection while capitalizing on pre-established infrastructure.
Introduction
Typical sandwich immunoassays indirectly detect the presence of an antigen using two antibodies. The capture antibody is bound to a solid surface and forms an antibody-antigen complex when in proximity to an appropriate antigen. A detection antibody is then introduced and binds to the antigen. After washing, the antibody/antigen/antibody complex remains and is detected by the labeled detection antibody as demonstrated in Figure 1A. Typical detection is done by a fluorescent or colorimetric detector, limiting multiplexing to 10 analytes due to broad spectral peaks2,3. In contrast, Raman-based systems have much narrower emission peaks resulting in enhanced multiplexing capabilities with sources claiming simultaneous detection of up to 100 analytes2,3.
Many literature sources are available which cover important aspects related to immunoassays4-6 such as step-by-step details to create personalized ELISA kits. Unfortunately, these protocols are for fluorescent or colorimetric detection, limiting multiplexing capability of customized immunoassays. To address this need, we present a detailed procedure to fabricate the UV-Vis/Raman immunoassay published previously7 for a direct immunoassay as illustrated in Figure 1B.
This protocol includes the fabrication of functionalized gold nanoparticle-based probes, illustrated in Figure 2. The procedure to make the Raman/UV-Vis probes begins by binding Raman reporters to the surface of gold nanoparticles (AuNPs). The AuNPs are then functionalized with antibodies that are associated with polyethylene glycol (PEG). Remaining binding sites on the AuNPs are blocked by binding methoxy polyethylene glycol thiol (mPEG-SH) to AuNPs to prevent subsequent non-specific binding during analysis. The prepared AuNP probes are tested by binding to antigens fixed to the wells of a polystyrene plate as illustrated in Figure 1B. Upon washing the plate, the AuNP probes are detected using UV-Vis spectroscopy while the associated Raman reporters are detected with Raman spectroscopy. Combining UV-Vis and Raman spectral data provides two methods of analyses, enhancing the capabilities of this immunoassay.
Protocol
1. Preparation of Buffers
- Phosphate Buffered Saline (PBS)
- Dilute 50 ml of 10x PBS with 450 ml HPLC grade water to make a 1x PBS concentration. Sterile filter the solution with a 0.22 µm filter.
- Store solution at room temperature.
- Preparation of Tris Buffered Saline + Tween 20 (TBST)
- Dilute 50 ml of 10x Tris Buffered Saline (TBS) with 450 ml HPLC grade water to make a 1x concentration. Add 250 μl of Tween-20 for a 0.05% (v/v) of Tween-20. Sterile filter the solution with a 0.22 μm filter.
- Store at room temperature.
- Preparation of Human Serum Albumin (HSA) Blocking Solution
- Weigh 0.45 g of HSA into 15 ml of sterile filtered 1x PBS to make a 3% w/v HSA solution. Vortex solution until HSA is fully dissolved.
- Store HSA solution at 4 °C.
NOTE: Bovine Serum Albumin (BSA) can also be used as a blocking solution.
- Preparation of PEGylated antibody (PEG-Ab) solution
NOTE: The antibody solution must be free from carrier or stabilizing proteins such as BSA, which would interfere with conjugation reactions by competing for the n-hydroxysulfosuccinimide (NHS) binding sites. If the antibody comes in a Tris or glycine buffer solution, it must undergo a buffer exchange to prevent amines or ammonium salts from interfering with the NHS conjugation reaction. If the antibody is in a lyophilized form, it can be resuspended according to the manufacturer's recommendation at a concentration of 1-10 mg/ml.- For antibodies in a Tris or glycine buffer, perform a buffer exchange to 100 mM sodium bicarbonate using a desalting column. Use the 100 mM buffer to raise the pH to approximately 8.5 to speed up the conjugation reaction.
- Hydrate ortho-pyridyl disulfide-PEG-NHS (OPSS-PEG-NHS) with 100 mM sodium bicarbonate to a volume of 1 ml at a concentration of 1 mg/ml or greater.
NOTE: OPSS-PEG-NHS should be made fresh and used within approximately 20 min. The NHS group on the OPSS-PEG-NHS has a half-life of approximately 20 min in an aqueous solution at pH 8.5. - Add OPSS-PEG-NHS to the antibody solution at a 2:1 ratio (PEG: Antibody) conjugation ratio to be used for the test samples. In a separate microcentrifuge tube, add OPSS-PEG-NHS to the antigen solution at a 2:1 conjugation ratio to be used for the control.
NOTE: The 2:1 ratio is assuming a 50% conjugation efficiency. The objective is to label each antibody with one PEG chain. In this step, over-labeling is better than under-labeling. Use the following equation to determine the appropriate volumes of OPSS-PEG-NHS and antibody solution:

where V is volume, C is concentration expressed in molecules or antibodies per ml. Subscripts PEG and Ab are OPSS-PEG-NHS and antibody, respectively. The final volume should be approximately 250 μl. - Incubate PEG-Ab solution at 4 °C for 8 hr or overnight. Store solution in working aliquots of approximately 25 μl at -20 °C to limit the freeze thaw cycles and make sure to use low binding tubes.
2. Prepare UV-Vis/Raman Probes
- Prepare bare AuNP solution
- Prepare a 2 ml solution of AuNPs with a concentration of approximately 1 x 1011 particles per ml.
- If the AuNPs need to be concentrated, fill low binding centrifuge tubes with 2,000 μl of stock AuNP and centrifuge at 5,000 x g for 20 min or until the supernatant is clear. Remove the supernatant by pipetting, being careful not to disturb the AuNP pellet.
- Combine the remaining AuNP solutions into one tube and estimate the concentration by obtaining a UV-Vis measurement and comparing values to known concentrations as this is a linear relationship.
- Prepare a 2 ml solution of AuNPs with a concentration of approximately 1 x 1011 particles per ml.
- Determine the appropriate Raman reporter labeling ratio
- Prepare a working solution of the Raman reporter dissolved in methanol. This concentration will be dependent on the reporter used. In this work, prepare 3,3′-diethylthiatricarbocyanine iodide (DTTC) at a working solution of 200 μM.
- Assuming a final volume of 100 μl for each well, add enough of the working reporter solution to each well of the first row of a 96-well plate such that the Raman reporter will range in concentrations from 0.2 μM to 10 μM. Add enough HPLC grade water to each well such that the volume is 80 μl. Add 20 μl of AuNP to each well making a final volume of 100 μl for each well. An example is provided in Table 1.
- Measure the UV-Vis spectra from 400 to 700 nm using a plate-reading UV-Vis spectrophotometer. The appropriate concentration is the highest concentration with defined peaks for the UV-Vis spectra. Repeat step 2.2.2 at increasing concentrations until the highest concentration ratio of Raman reporters to AuNPs is found.
NOTE: The dye and the AuNP shape, size, and manufacturer influence the appropriate concentration. Therefore, the steps listed must be evaluated and altered depending on the components used. This protocol involved the use of a positively charged dye. As such, binding between the AuNP and reporter was improved by using negatively charged AuNPs. This was done by using citrate capped AuNPs. See the Discussion section for further details.
- Binding Raman reporter and PEG-Ab to AuNP
- Prepare two 1.5 ml batches of AuNP and Raman reporter at the previously determined concentration, allowing the Raman reporter to bind to the AuNPs for 30 min at room temperature.
- Add the PEGylated antibody (PEG-Ab) to one batch of the AuNP and Raman reporter solution to create a 200:1 ratio of antibodies to particles. This solution will be for the test samples. In a separate microcentrifuge tube, add the PEGylated antigen to the other batch of the AuNP and Raman reporter solution at a 200:1 ratio of antibody to particles to be used as the control. Incubate the solutions for 30 min at room temperature.
NOTE: The ratio of antibodies to particles will be specific to the AuNPs and dye used and should be optimized for each individual case. The objective here is to have the highest ratio of antibodies for the AuNP probes to bind to while preventing aggregation of the particles. Use the following equation to determine the appropriate volumes to add together:

where V is volume, C is concentration expressed in particles or antibodies per ml. The final volume should be approximately 1.5 ml.
- Block remaining sites on the AuNP surface with mPEG-SH.
- Prepare mPEG-SH by dissolving solid methoxy polyethylene glycol thiol to a 200 μM concentration using water. Vortex the solution until mPEG-SH is completely dissolved.
- Add mPEG-SH at a 40,000:1 ratio to the AuNP-PEG-Ab solution made in step 2.3. Incubate the solution at room temperature for 10 min to ensure the remaining sites on the gold nanoparticle are blocked. Use the following equation to determine the appropriate volumes to add together:
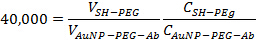
where V is volume, C is concentration expressed in particles or antibodies per ml. The final volume should be approximately 1.5 ml.
- Recover functionalized Raman probes.
- Centrifuge particles at 5,000 x g for 20 min in low bind centrifuge tubes or until the supernatant is clear. Remove the supernatant by pipetting being careful not to disturb the AuNPs.
- Resuspend the particles with 1 ml of 1x PBS solution that was made previously. Estimate the AuNP concentration by taking a UV-Vis measurement of a small volume of solution (3 μl) and compare the results to measurements from a known AuNP concentration. Adjust the volume such that the final solution is at least 1 x 1011 particles per ml.
- Store solutions at 4 °C until it is used for functionalizing of the immunoassay plate. Use the solutions within one week.
| Volumes to add of each component (ml) | |||
| DTTC final concentration (mM) | DTTC working solution (200 mM) | AuNP | Water |
| 0.2 | 0.1 | 20 | 79.9 |
| 0.6 | 0.3 | 20 | 79.7 |
| 1 | 0.5 | 20 | 79.5 |
| 2 | 1.0 | 20 | 79 |
| 5 | 2.5 | 20 | 77.5 |
| 7 | 3.5 | 20 | 76.5 |
| 10 | 5.0 | 20 | 75 |
Table 1. DTTC dilution example. Various dilutions of DTTC and the associated volumes of stock DTTC, gold nanoparticle solution, and water.
3. Immunoassay Plate Preparation
- Bind desired antigen to the immunoassay plate.
- Prepare enough diluted antigen (50 μg/ml) to fill the polystyrene wells. Vortex the solution, and immediately add the solution to the plate wells. Allow the antigen to bind to the plates for 1 hr at room temperature.
- Wash off unbound antigens.
- Remove the excess antigen solution by dumping solution into a disposal container and hitting the plate against a paper-towel-covered tabletop.
- Add TBST to the wells to wash the surface then remove the wash in the same manner as stated previously. Repeat this step two more times.
- Block remaining binding sites on the plate to prevent non-specific binding.
- Add 70 μl of HSA blocking solution to each well of the plate and incubate at room temperature for 30 min.
- Remove and rinse the plate using the same procedure as specified in step 3.2. Cover the plate and store dry at 4 °C until ready for further use.
- Functionalize immunoassay plate.
- Add 70 μl of the probe nanoparticles prepared in Section 2 to the first column of a 96-well plate and dilute subsequent columns using a 1:2 serial dilution. Allow the plate to incubate for at least 1 hr. An example of how to prepare the immunoassay plate is given in Figure 3.
- Wash the plate with TBST five times as detailed in steps 3.2, making sure to dispose of the AuNPs appropriately. After the final wash, add 70 μl of 1x PBS to each well and cover with a plate seal.
NOTE: The control samples should be clear. If non-specific binding has occurred, the control samples will have a similar color as the test samples.
- Test assay sensitivity by UV-Vis and Raman spectroscopy.
- For each well, measure the UV-Vis spectra ranging from 400 to 700 nm using a plate-reading UV-Vis spectrophotometer.
- Using an inverted Raman microscope, focus the objective onto the surface of the well that has the AuNP probes. Obtain a Raman spectra of the well. Collect a spectrum ranging from 1,800 cm-1 to 400 cm-1. Repeat this step for all wells.
- Using an appropriate spectral software, perform an 11th order polynomial baseline correction for the Raman spectra and a 3rd order polynomial for the UV-Vis spectra.
- Using an appropriate spectral software, normalize the Raman and UV-Vis spectra. Set the maximum value to 1 and scale all other values accordingly. To normalize the Raman spectra, select a unique polystyrene peak and set it equal to 1 and scale all other values accordingly.
- Using an appropriate spectral software, perform peak integration for each spectrum. For Raman spectra, the peak representing the Raman reporter must be in a region absent of polystyrene peaks. To perform peak integration, specify the integral boundaries for the desired peak and record the desired peak area for all samples including the controls.
- Plot the average peak area of interest as a function of the log of the AuNP concentration with error bars for each point indicating its associated standard deviation. Fit these calibration points to a 4-parameter logistic curve.
- Determine the mean value of the blank by averaging the area of the peak of interest for a blank sample. Determine the standard deviation of these areas; this is the standard deviation of the blank.
- For the same peak analyzed in the previous step, find the standard deviation of that peak area for the lowest concentration.
- Calculate the limit of the blank and lower limit of detection as specified in the Representative results section. Use these values with the 4PL calibration curves to determine the LLOD in terms of AuNP concentration.
Results
In this study, 60 nm gold particles were used for UV-Vis spectroscopy. UV-Vis absorption spectra from 400 to 700 nm were collected and the peak areas for each AuNP concentration were determined using an open source spectral analysis software8. Prior to peak integration, the collected spectra underwent baseline correction using a three-point polynomial fit. Peak areas were used to generate a logarithmic calibration curve as demonstrated in Figure 4. It should be noted that Figures 4
Discussion
In the detailed protocol, there are several critical points to address. One issue is the choice of Raman reporter and gold nanoparticle. Although the protocol was written to be adapted for individual use, the Raman reporter DTTC was used as an example. DTTC is a positively charged reporter and binds to negatively charged surfaces such as citrate capped AuNPs. This protocol can be adapted for negatively charged reporters by using gold nanoparticles with a positive surface charge. For example, polyethyleneimine (PEI) cappe...
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
This work was supported by a Research Catalyst Award from Utah State University. The authors would like to thank Annelise Dykes, Cameron Zabriskie, and Donald Wooley for their contributions.
Materials
| Name | Company | Catalog Number | Comments |
| 60 nm Gold Nanoparticle | Ted Pella, Inc. | 15708-6 | These are citrate capped gold nanoparticles. Please see Discussion for relationship between Raman reporter and AuNP surface charge and its imporance to proper selection of AuNP and/or Raman reporter. |
| Sodium Bicarbonate | Fisher Scientific | S233-500 | |
| Methanol | Pharmco-Aaper | 339000000 | |
| Tris Buffered Saline (10x) pH 7.5 | Scy Tek | TBD999 | |
| Bottle Top Filtration Unit | VWR | 97066-202 | |
| Tween 20 (polysorbate 20) | Scy Tek | TWN500 | Used as an emulsifying agent for washing steps. |
| Phosphate Buffered Saline 10x Concentrate, pH 7.4 | Scy Tek | PBD999 | |
| Protein LoBind Tube 2.0 ml | Eppendorf Tubes | 22431102 | LoBind tubes prevent binding of proteins and AuNPs to surfaces of the tubes. |
| Protein LoBind Tube 0.5 ml | Eppendorf Tubes | 22431064 | LoBind tubes prevent binding of proteins and AuNPs to surfaces of the tubes. |
| Microplate Devices UniSeal | GE Healthcare | 7704-0001 | Used for sealing and storing functionalized plates. |
| Assay Plate, With Low Evaporation Lid, 96 Well Flat Bottom | Costar | 3370 | |
| HPLC grade water | Sigma Aldrich | 270733-4L | |
| 3,3′-Diethylthiatricarbocyanine iodide (DTTC) | Sigma Aldrich | 381306-250MG | Raman reporter |
| mPEG-Thiol, MW 5,000 - 1 gram | Laysan Bio, Inc. | MPEG-SH-5000-1g | |
| OPSS-PEG-SVA, MW 5,000 - 1 gram | Laysan Bio, Inc. | OPSS-PEG-SVA-5000-1g | OPSS-PEG-SVA has an NHS end. |
| Mouse IgG, Whole Molecule Control | Thermo Fisher Scientific | 31903 | Antigen |
| Goat anti-Mouse IgG (H+L) Cross Adsorbed Secondary Antibody | Thermo Fisher Scientific | 31164 | Antibody |
| Human Serum Albumin Blocking Solution | Sigma Aldrich | A1887-1G | Bovine serum albumin can be used instead. |
| Mini Centrifuge | Fisher Schientific | 12-006-900 | |
| UV-Vis Spectrophotometer | Thermo Scientific | Nanodrop 2000c | |
| UV-Vis Spectrophotometer | BioTek | Synergy 2 | |
| Desalting Columns | Thermor Scientific | 87766 | |
| In-house built 785 nm inverted Raman microscope unit | N/A | N/A | An inverted Raman microscope is best for proper focusing onto surface of the well plate. Otherwise a very low magnification will be used due to height of the 96-well plate. An in-house built system was used as it was cheaper than buying from a vendor. However, any commercially available inverted Raman microscope system can be used. |
References
- Israelsen, N. D., Hanson, C., Vargis, E. Nanoparticle properties and synthesis effects on surface-enhanced Raman scattering enhancement factor: an introduction. Sci. World J. , e124582 (2015).
- Wang, Y., Schlücker, S. Rational design and synthesis of SERS labels. Analyst. 138 (8), 2224-2238 (2013).
- Wang, Y., Yan, B., Chen, L. SERS tags: novel optical nanoprobes for bioanalysis. Chem. Rev. 113 (3), 1391-1428 (2013).
- . . The Immunoassay Handbook: Theory and applications of ligand binding, ELISA and related techniques. , (2013).
- Cox, K. L., Devanarayan, V., Kriauciunas, A., Manetta, J., Montrose, C., Sittampalam, S. Immunoassay Methods. Assay Guid. Man. , (2004).
- . . ELISA development guide. , (2016).
- Israelsen, N. D., Wooley, D., Hanson, C., Vargis, E. Rational design of Raman-labeled nanoparticles for a dual-modality, light scattering immunoassay on a polystyrene substrate. J. Biol. Eng. 10, (2016).
- Menges, F. . Spekwin32 - optical spectroscopy software. Version 1.72.1. , (2016).
- Findlay, J. W. A., Dillard, R. F. Appropriate calibration curve fitting in ligand binding assays. AAPS J. 9 (2), E260-E267 (2007).
- Yu, X. Quantifying the Antibody Binding on Protein Microarrays using Microarray Nonlinear Calibration. BioTechniques. 54, 257-264 (2013).
- Armbruster, D. A., Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 29 (Suppl 1), S49-S52 (2008).
- . . EP17-A2: Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures; Approved Guideline. 32. No 8, (2012).
- Leigh, S. Y., Som, M., Liu, J. T. C. Method for assessing the reliability of molecular diagnostics based on multiplexed SERS-coded nanoparticles. Plos One. 8 (4), e62084 (2013).
- Sinha, L. Quantification of the binding potential of cell-surface receptors in fresh excised specimens via dual-probe modeling of SERS nanoparticles. Sci. Rep. 5, 8582 (2015).
- Shi, W., Paproski, R. J., Moore, R., Zemp, R. Detection of circulating tumor cells using targeted surface-enhanced Raman scattering nanoparticles and magnetic enrichment. J. Biomed. Opt. 19, 056014 (2014).
- Xia, X., Li, W., Zhang, Y., Xia, Y. Silica-coated dimers of silver nanospheres as surface-enhanced Raman scattering tags for imaging cancer cells. Interface Focus. 3 (3), 20120092 (2013).
- McLintock, A., Cunha-Matos, C. A., Zagnoni, M., Millington, O. R., Wark, A. W. Universal surface-enhanced Raman tags: individual nanorods for measurements from the visible to the infrared (514-1064 nm). Acs Nano. 8 (8), 8600-8609 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved