Method Article
Automation of a Positron-emission Tomography (PET) Radiotracer Synthesis Protocol for Clinical Production
* These authors contributed equally
In This Article
Summary
Positron-emission tomography (PET) imaging sites that are involved in multiple early clinical research trials need robust and versatile radiotracer manufacturing capabilities. Using the radiotracer [18F]Clofarabine as an example, we illustrate how to automate the synthesis of a radiotracer using a flexible, cassette-based radiosynthesizer and validate the synthesis for clinical use.
Abstract
The development of new positron-emission tomography (PET) tracers is enabling researchers and clinicians to image an increasingly wide array of biological targets and processes. However, the increasing number of different tracers creates challenges for their production at radiopharmacies. While historically it has been practical to dedicate a custom-configured radiosynthesizer and hot cell for the repeated production of each individual tracer, it is becoming necessary to change this workflow. Recent commercial radiosynthesizers based on disposable cassettes/kits for each tracer simplify the production of multiple tracers with one set of equipment by eliminating the need for custom tracer-specific modifications. Furthermore, some of these radiosynthesizers enable the operator to develop and optimize their own synthesis protocols in addition to purchasing commercially-available kits. In this protocol, we describe the general procedure for how the manual synthesis of a new PET tracer can be automated on one of these radiosynthesizers and validated for the production of clinical-grade tracers. As an example, we use the ELIXYS radiosynthesizer, a flexible cassette-based radiochemistry tool that can support both PET tracer development efforts, as well as routine clinical probe manufacturing on the same system, to produce [18F]Clofarabine ([18F]CFA), a PET tracer to measure in vivo deoxycytidine kinase (dCK) enzyme activity. Translating a manual synthesis involves breaking down the synthetic protocol into basic radiochemistry processes that are then translated into intuitive chemistry "unit operations" supported by the synthesizer software. These operations can then rapidly be converted into an automated synthesis program by assembling them using the drag-and-drop interface. After basic testing, the synthesis and purification procedure may require optimization to achieve the desired yield and purity. Once the desired performance is achieved, a validation of the synthesis is carried out to determine its suitability for the production of the radiotracer for clinical use.
Introduction
An increasing array of biological targets can be dynamically visualized in living subjects via the molecular imaging modality PET. PET provides in vivo assays of specific biological, biochemical, and pharmacological processes by using specific radiotracers (molecules labeled with positron-emitting radionuclides) that are injected into the subject prior to imaging1. The increased use of PET to study a wide variety of these processes in basic science and clinical research2,3,4, and in the discovery, development, and clinical use of drugs in patient care5,6, is leading to a growing demand for diverse radiotracers7,8. To avoid radiation exposure to the radiochemist and to ensure a reproducible production of these short-lived tracers, they are typically manufactured using an automated radiosynthesizer operating inside a "hot cell". Recent radiosynthesizers use a disposable-cassette/kit architecture to simplify the task of complying with clinical-grade manufacturing while also providing the flexibility to prepare multiple types of radiotracers simply by swapping out cassettes9. However, in early clinical stages, there are usually no commercially-available cassettes/kits to perform the automated radiosynthesis; consequently, PET drug manufacturing facilities struggle to customize systems to implement cGMP-grade tracer production capabilities within a suitable timeframe and at a reasonable cost. Thus, radiosynthesizers have been developed that combine the cassette/kit architecture with features to facilitate the development and optimization of tracers.
The ELIXYS FLEX/CHEM (ELIXYS) is an example of a flexible cassette-based radiosynthesizer with a wide reagent, solvent, and reaction temperature compatibility10. It has three reaction vessels and uses a robotic mechanism to dynamically configure the fluid pathway as required by any particular synthesis protocol11. The synthesizer software allows the creation of synthesis programs (Sequences) for different tracers by dragging and dropping Unit Operations such as Trap Isotope, Elute Isotope, Add Reagent, React, and Evaporate12. Each unit operation has a variety of programmable parameters available to the operator, such as Duration, Temperature, or inert gas driving pressure (Pressure). By understanding the nature of each unit operation, a manual synthesis can be readily translated into a sequence of unit operations and then be modified during the optimization of the protocol13. In combination with the ELIXYS PURE/FORM module, the integrated system can also perform an automated purification and formulation of the PET tracer. Using this radiosynthesizer, we have previously reported the automated synthesis of 24 different 18F-labeled tracers and prosthetic groups11,14,15,16, as well as the automated enzymatic radiofluorination of biomolecules17, by simply changing reagents and not the configuration of the system. Others have shown the automated synthesis of [18F]RO6958948 for the imaging of tau neurofibrillary tangles18, the automated synthesis of the prosthetic group [18F]F-Py-TFP with a subsequent labeling of peptides19, and the automated synthesis of [18F]AM580 for the imaging of phosphodiesterase 10a (PDE10A)20. Furthermore, several groups have shown the production of tracers suitable for clinical use, including 4-[18F]Fluorobenzyl-triphenylphosphonium ([18F]FBnTP) for the imaging of mitochondrial membrane potential21, [18F]DCFPyL for the imaging of prostate-specific membrane antigen (PSMA)22, and [18F]THK-5351 for the imaging of tau23.
In this paper, we use our experience with [18F]CFA to illustrate how a manual radiosynthetic procedure can be straightforwardly and rapidly translated into an automated synthesis suitable for routine production following cGMP guidelines. The tracer [18F]CFA was designed for the imaging of dCK activity. The manual radiosynthesis of [18F]CFA was originally described by Shu et al.24 as a procedure using two reaction vessels, intermediate silica cartridge purification, and a final HPLC purification step (see Supplementary Material, Section 1 for details). Recent in vitro and preclinical studies have shown the exceptional specificity of this tracer to dCK, and first-in-human studies have shown favorable biodistribution25. There is an immediate interest in wider-scale clinical studies to confirm the sensitivity of [18F]CFA PET to variations in dCK activity and a longer-term interest in the potential clinical applications of this tracer26. It may be a useful biomarker for therapies that trigger T-cell activation, induce DNA damage, or rely on dCK-dependent nucleoside analog prodrugs. In particular, [18F]CFA may enable the stratification of patients for a potential response to treatment with Clofarabine. [18F]CFA may also facilitate the study and development of dCK inhibitors that are advancing toward clinical trials. Since this tracer has traditionally been synthesized manually, advancing all of these studies requires a reliable, automated synthesis of [18F]CFA suitable for clinical use.
Although we previously reported an automated synthesis of [18F]CFA for preclinical studies16, this protocol builds further on these efforts and describes additional modifications needed for the clinical production of this tracer, including the integration of fully-automated purification and formulation, protocol validation, and quality-control testing. The general procedures described here are not limited to developing an automated and clinically-suitable synthesis of [18F]CFA but can be generalized in a straightforward manner to develop automated syntheses suitable for clinical use of other radiotracers labeled with fluorine-18.
Protocol
1. General Procedure for the Automation and Validation of a Radiosynthesis Protocol for Clinical Manufacturing
- Analyze the eligibility of the manual synthesis scheme for clinical manufacturing
- Perform risk analysis of product contamination with any undesired residual chemicals.
- Avoid Class 1 solvents such as benzene and replace them with appropriate alternative solvents (Class 2 or Class 3).
- Avoid chemicals that would be difficult to detect in the final formulation as potential residual impurities.
- Choose only chemicals that are commercially available in high purity grade (the USP or Ph.Eur. grade desired) and are provided with a certificate of analysis.
- Refine the synthesis scheme if any undesirable chemicals or solvents are detected by the risk analysis and repeat section 1.1 until none remain.
- Perform risk analysis of product contamination with any undesired residual chemicals.
- Automate the synthesis protocol
- If an automated protocol for the tracer using the same synthesizer has already been created and uploaded to an online repository, download a copy of the synthesis program.
- If an automated synthesis program does not already exist, create one.
- Using paper and pen, divide the manual synthesis into high-level steps (e.g., drying/activating [18F]fluoride, heating to facilitate a radiochemical reaction, performing a purification step, etc.). Further break down the high-level steps into discrete, basic processes that are required. As an example, the synthesis scheme of [18F]CFA is shown in Figure 1, the identification of high-level steps is shown in Figure 2A, and the breakdown into processes is shown in Figure 2B.
- Using paper and pen, map each process into the individual unit operations provided by the synthesizer software. As an example, an analysis of the mapping of basic processes in the synthesis of [18F]CFA to suitable unit operations in the synthesizer software13 is shown in Figure 2C.
- Using the radiosynthesizer programming interface, create a blank program and append each of the identified unit operations in sequence by clicking the Menu button (top left) and selecting Sequences, and then clicking the New Sequence button. For each unit operation identified in step 1.2.2.2, drag the unit operation from the available operations to the filmstrip view and click or type to fill out the desired value of each parameter of the unit operation. Figure 3 shows an example of the interface when all the operations to synthesize [18F]CFA have been populated, and the user has selected the first REACT unit operation to edit parameter values. The final synthesis program for [18F]CFA is described in the Supplementary Material, Tables S1 and S2.
- Verify the synthesis program.
- Perform a dry run. Set up and run the program as in steps 2.1 - 2.3, using all reagents and solvents other than the radionuclide (e.g., [18F]fluoride) to verify expected behavior.
- If necessary, adjust the unit operation parameter values in the program (e.g., the time or driving pressure to completely transfer a reagent, the time/temperature to evaporate a solvent to the desired level, etc.), and retest. To adjust parameter values, first, return to the Edit mode by choosing Sequences from the main menu (top left) and select the newly created program. Next, click on the desired unit operation in the filmstrip view (bottom of the screen), navigate to the desired parameter, and select or type the new value.
- Perform a low-activity (< 370 MBq) test run to evaluate the program.
- Optimize the automated synthesis by adjusting parameter values to improve the yield, synthesis time, repeatability, and any other desired measurable outcome.
- Develop quality control (QC) testing procedures
- Using a non-radioactive reference of the final product and samples of potential chemical impurities, develop an analytical radio-HPLC and/or radio-thin layer chromatography (radio-TLC) method with sufficient separation of species for the determination of chemical purity, molar activity, radiochemical purity, and radiochemical identity. Validate the analytical method(s) for repeatability and linearity and determine the detection and quantification limits.
- Similarly, develop and validate a gas chromatography method to analyze volatile impurities (e.g., residual amounts of solvents that are used during the synthesis).
- Develop and validate analytical assays that allow the detection and quantification of other potential impurities (e.g., cryptand 222 via the standard color spot test).
- Use standard procedures for the determination of sterility, pH, radionuclidic identity, radionuclidic purity, radioactivity concentration, product volume, and endotoxin levels.
- Perform synthesis validation
- Establish standard operating procedures (SOPs) for the synthesis and QC testing procedures and integrate a materials and equipment tracking system compliant with current good manufacturing practice (cGMP) requirements.
- Validate the synthesis procedures via three independent and consecutive production runs at the same radioactivity levels as intended for clinical manufacturing following the SOPs. Document the synthesis performance and results of QC testing.
- All consecutive validation runs must pass the pre-set QC limits. If a validation run fails, repeat the whole validation process after appropriately addressing the root cause of the failure.
2. Example: Automated Synthesis of [18F]CFA for Clinical Use
- Prepare the radiosynthesizer
- Power on the radiosynthesizer.
- Ensure the inert gas supply is turned on with sufficient pressure and that the necessary valves are open so that the radiosynthesizer is connected to the gas supply.
- Install new disposable cassettes in Reactor #1 and #2 positions and insert reaction vessels containing magnetic stir bars. Ensure that each cassette transfer dip tube is pointed straight down.
- Prepare reagent vials and install them in the cassettes according to the diagram in Figure 4.
- Install an empty [18O]H2O recovery vial in the W1 position of Cassette #1.
- Activate a quaternary methylammonium (QMA) cartridge by first passing 12 mL of a 1 M KHCO3 solution through it, followed by 12 mL of deionized water. Condition a silica Sep-Pak cartridge by passing 5 mL of ethyl acetate through it.
- Connect the cartridges and make all cassette tubing connections as shown in Figure 5A. Verify that no cassette tubing (including unused tubing) hangs in the interior, where it may interfere with robotic movements.
- Connect the [18F]fluoride source line from the cyclotron to the [18F]fluoride input line on Cassette #1.
- Ensure the waste container is empty. Place waste lines from the purification/formulation subsystem to the waste container (i.e., sample loop 1 waste line, HPLC subsystem waste line, and the syringe pump waste line).
- Connect the HPLC input lines. Place HPLC mobile phase input line “A” in a container of 25 mM ammonium acetate and HPLC mobile phase input line “B” in a container of EtOH.
- Equilibrate the purification/formulation subsystem and HPLC column.
- Open the control page for the purification/formulation module in the software by selecting HPLC from the main menu (top left). By default, the Purification tab will already be selected. (This page is shown in Figure 6.)
- Set the flow rate to 5.0 mL/min at the defined solvent composition and choose which column position the purification column is installed in. Turn on the HPLC pump in the isocratic mode for at least 10 min.
- Rinse the product line and all fraction collection lines with the mobile phase, each for 1 min.
- Rinse each HPLC sample loop and HPLC sample loop transfer tubing with 10 mL of the mobile phase using a syringe.
- Connect the purification/formulation subsystem syringe pump input lines. Use concentrated sodium chloride (90 mg/mL) for the Elute line and 0.9% saline for the Reconstitute line.
- Prime the formulation subsystem.
- Navigate to the Formulation tab of the purification/formulation control page.
- To prime the concentrated sodium chloride (90 mg/mL), select the Elute tab. Press Initialize to initialize the syringe pump. Dispense 5 mL.
- To prime the 0.9% saline, select the Reconstitute tab. Dispense 5 mL.
- Connect the Product and Final product lines from the front of the purification/formulation subsystem in a T-connection. Connect the output of the T-connection to a sterile filter (0.22 µm) which, in turn, is connected to the final sterile product vial. Insert a vent needle with a sterile filter into the headspace of the final product vial. A photograph of the final system set-up is shown in Figure 5B.
- Add dry ice and EtOH or MeOH to the cold trap.
- Run the synthesis program
- Navigate to the list of programs by selecting Sequences from the main menu button (top left). Select the [18F]CFA program and start the program by pressing the Run button.
- Carefully go through each item on the pre-run checklist and check them off as they are completed. Part of the pre-run checklist screen is shown in Figure 7.
- Press Continue to confirm the setup is complete and cause the automated synthesis to begin.
- If desired, monitor the synthesis in real-time via visual feedback (reactor cameras), sensor readings (e.g., temperature, pressure, vacuum, radiation reading, etc.) and countdown timers. A representative screenshot is shown in Figure 8.
- During the Purification unit operation, select Product when the product peak has begun to appear on the radiation detector chromatogram. A representative screenshot during this unit operation (containing a chromatogram of the UV detector and radiation detector output) is shown in Figure 9.
- Once the radiation detector chromatogram peak has returned to the baseline, select Waste to divert the flow path of the HPLC subsystem to the waste container.
- Set up and run the Formulation program
- From the list of programs (Sequence screen), open the [18F]CFA Formulation program.
- Adjust the parameters of the formulation unit operation.
- Calculate the volume of the collected product fraction (Vfraction) based on the HPLC pump flow rate and the duration of the fraction collection.
- Calculate the volume of additional sodium chloride (90 mg/mL) required to achieve isotonicity and calculate the amount of additional saline required to dilute the EtOH concentration below 10%.
- Modify the program with these values. The volume of sodium chloride (90 mg/mL) is entered for the Elute step and the volume of saline is entered for the Reconstitute step. (The calculations are described in the Supplementary Material, Figure S2.)
- Save the program.
- Run the program. The system will dilute the collected purified product fraction with sodium chloride and saline to ensure the isotonicity of the formulation and deliver it through a sterilizing filter into the sterile product vial.
- Collect formulated [18F]CFA for quality control and shipment
- Remove the formulated [18F]CFA product from the hot cell.
- Using sterile working techniques, withdraw two samples (300 µL) to perform quality control tests.
- Use the first sample to test for sterility of the final formulation by inoculating fluid thioglycolate media and tryptic soy broth for 14 d without observing any growth.
- Use the second sample to perform quality control according to the procedures developed in step 1.3. The procedures established at the UCLA Ahmanson Biomedical Cyclotron Facility in accordance with the U.S. Pharmacopeia are described below.
- Assess appearance by visual inspection.
- Assess pH with an indicator paper.
- Assess bacterial endotoxin content using a kinetic chromogenic Bacterial Endotoxin Test (BET).
- Assess radiochemical identity with analytical radio-HPLC by verifying the co-elution of the radioactive sample and a non-radioactive reference compound.
- Assess radiochemical purity with analytical radio-HPLC by comparing the area under the curve (AUC) of radioactive impurities in the gamma-detector chromatogram with the AUC corresponding to the desired product.
- Assess chemical purity with analytical HPLC by determining the AUC in the UV-detector chromatogram of all UV-active impurities.
- Assess molar activity and carrier mass with analytical radio-HPLC by determining the AUC corresponding to the desired product in the UV-detector chromatogram.
- Assess the half-life of the probe by measuring its activity at two different timepoints and fitting a decay curve.
- Assess the residual solvent content of the formulation by gas chromatography.
- Assess the radionuclide energy using a gamma spectrometer.
- Assess the cryptand 222 content using a TLC-based spot test.
- If all tests pass, release the probe formulation for shipment to the clinical imaging site.
- Post-run and system shutdown
- Rinse the HPLC purification column and all tubing used for product collection with 70% (v/v) EtOH in water. This should be done with the PURE/FORM Control page, similar to step 2.1.12.
- Shut down the radiosynthesizer via the Power button on the software. A pop-up window will indicate when the power to the system can be turned off.
- Turn off the compressed air and inert gas supplies by closing the appropriate shutoff valves.
- Allow time for residual radioactivity in the hot cell to decay (typically overnight).
- Clean the radiosynthesizer
- Remove and dispose of all cassettes, cartridges, reactor vials, and reagent vials used during the synthesis.
- Empty the contents of the cold trap.
- Clean the purification subsystem fluid paths.
- Open an existing cleaning program or create a new program which contains one Purification unit operation in cleaning mode (i.e., with the Clean checkbox selected). See Supplementary Material, Figure S9 for an example.
- On the parameter configuration page, select the column that was used for the purification and the HPLC mobile phase input line that is connected to a bottle containing 70% EtOH in water. Program a flow rate of 2 mL/min, a rinsing duration for each injection loop of 5 min, and a rinsing duration for each product and fraction output of 30 s. Select Dry Lines and program a duration of 30 s.
- Place all fraction line outputs in a large waste container.
- Run the program.
- After completion, empty the waste container.
- Clean the formulation subsystem fluid paths.
- Open an existing program or create a new program which contains one Formulation unit operation in cleaning mode (i.e., with the Clean checkbox selected under the Clean tab). See Supplementary Material, Figure S10 for an example.
- Fill a clean dilution reservoir (at the front of the purification/formulation subsystem) with 100 mL of EtOH.
- Place the purification/formulation subsystem Elute input line in an EtOH reservoir (containing > 50 mL of EtOH).
- Place the Rinse and Reconstitute input lines in a waste container along with the final product output line.
- Run the program.
- After completion, empty the waste container.
Results
A method to automate the production of [18F]CFA was developed and three validation batches were synthesized. Synthesis, purification, and formulation of [18F]CFA was achieved in 90 ± 5 min (n = 3) and the non-decay-corrected radiochemical yield was 8.0 ± 1.4% (n = 3). The activity yields of the three runs were 3.24 GBq, 2.83 GBq, and 3.12 GBq, starting from 34.3 GBq, 41.8 GBq, and 41.1 GBq, respectively. The obtained [18F]CFA formulations passed all quality control tests (Table 1). The automated protocol is currently being used for the production of clinical-grade [18F]CFA to support clinical trials.
| Quality control data | Validation run 1 | Validation run 2 | Validation run 3 |
| [requirement for “Pass”] | |||
| Appearance | Pass | Pass | Pass |
| [clear, colorless, free of particulate matter] | |||
| Radioactivity concentration at EOS | 213 MBq/mL | 210 MBq/mL | 180 MBq/mL |
| [≤ 740 MBq/mL @ EOS] | |||
| pH | 6 | 5.8 | 6 |
| [5.0 – 8.0] | |||
| Half-life | 115 min | 108 min | 112 min |
| [105 – 115 min] | |||
| Radiochemical purity | 99% | 99% | 99% |
| [> 95%] | |||
| Radiochemical identity by relative retention time (RRT) | 1.01 | 1.01 | 1.01 |
| [1.00 < RRT < 1.10] | |||
| Molar activity | 314 GBq/µmol | >370 GBq/µmol | >370 GBq/µmol |
| [≥ 3.7 GBq/µmol] | |||
| Total carrier mass in final product | 3.1 µg | <1 µg | <1 µg |
| [≤ 50 µg/dose] | |||
| Total impurity mass in final product | ND | ND | ND |
| [≤ 1 µg / dose] | |||
| Maximum allowable injection volume based on total carrier mass ≤ 50 µg/dose AND total impurity mass ≤ 1 µg/dose | Whole batch | Whole batch | Whole batch |
| Residual EtOH content by GC | 8.90% | 9.50% | 9.60% |
| [≤ 10%] | |||
| Residual EtOAc content by GC | <1 ppm | <1 ppm | <1 ppm |
| [≤ 5000 ppm] | |||
| Residual MeCN content by GC | <1 ppm | <1 ppm | <1 ppm |
| [≤ 410 ppm] | |||
| Residual K222 by color spot test | Pass | Pass | Pass |
| [< 50 µg/mL] | |||
| Filter membrane integrity test | Pass | Pass | Pass |
| [bubble point ≥ 50 psi] | |||
| Bacterial endotoxins | Pass | Pass | Pass |
| [≤ 175 EU/batch] | |||
| Radionuclidic purity by gamma spectroscopy | Pass | Pass | Pass |
| [>99.5%] | |||
| Sterility | Pass | Pass | Pass |
| [meet USP <71> requirements] |
Table 1: Quality control (QC) test data summary for three validation batches. EOB = end of bombardment; EOS = end of synthesis; ND = not detected.
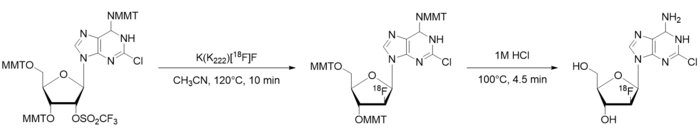
Figure 1: [18F]CFA radiosynthesis scheme. MMT = Monomethoxytrityl. Please click here to view a larger version of this figure.
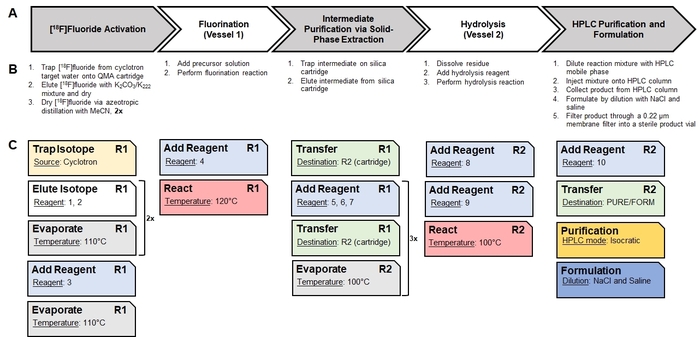
Figure 2: Translation of a manual synthesis into an automated sequence of unit operations. (A) This panel gives an overview of the high-level steps in the manual synthesis of [18F]CFA. (B) This panel shows the basic procedures needed to perform each of the high-level steps. (C) Radiosynthesizer-specific unit operations used to perform the basic procedures are shown as cards. Each unit operation has its own set of parameter values (shown as underlined) which are configured through the software. The notation "R1" and "R2" indicate the reaction vessels #1 and #2, respectively. The reagents corresponding to the reagent numbers are identified in Figure 4. The series of unit operations is saved as a Sequence and executed by the software to perform the automated synthesis. Please click here to view a larger version of this figure.
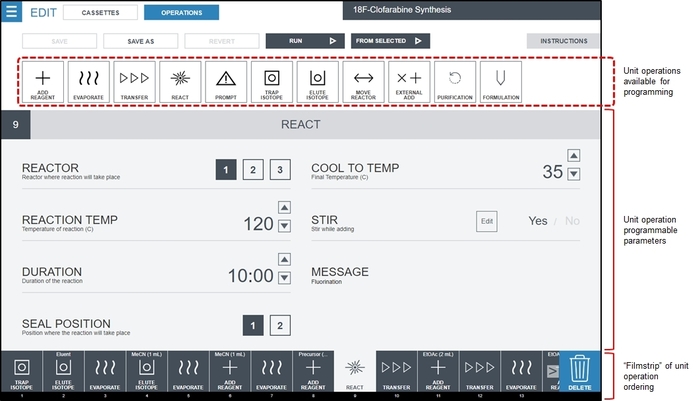
Figure 3: Screenshot of the radiosynthesizer (ELIXYS) software interface to create a synthesis program. Unit operations are placed in the desired order in the Filmstrip using a drag-and-drop interface. In this screenshot, a React unit operation is selected, and its editable parameter values are shown in the main part of the screen. In this example, the fluorination reaction will be carried out in reaction vessel #1 (sealed) at 120 °C for 10 min with active stirring. The vessel will be cooled to 35 °C after the reaction time has elapsed. Details of parameter values that can be programmed for other unit operations are shown in the Supplementary Material, Section 3. Please click here to view a larger version of this figure.
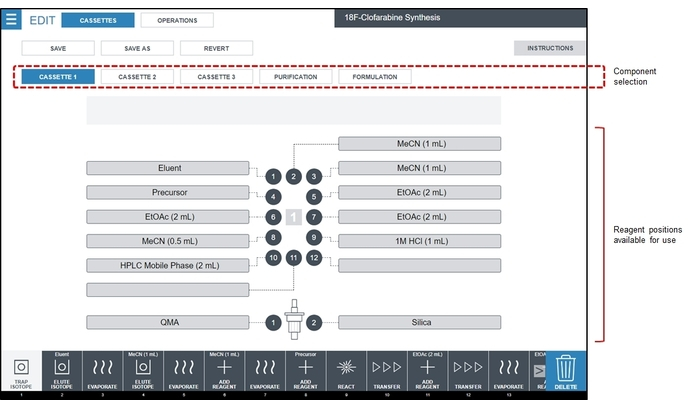
Figure 4: Screenshot of the reagent configuration screen. For the [18F]CFA synthesis sequence, all reagents are loaded into disposable cassette #1, which is shown highlighted in the component selection area. For the [18F]CFA synthesis described here, Eluent is 1.0 mg of K2CO3 + 5.0 mg of K222 in 0.4 mL of H2O/0.5 mL of MeCN, Precursor is 6 mg of CFA precursor in 0.6 mL of MeCN, and HPLC Mobile Phase is 85:15 v/v 25 mM ammonium acetate:ethanol. Please click here to view a larger version of this figure.
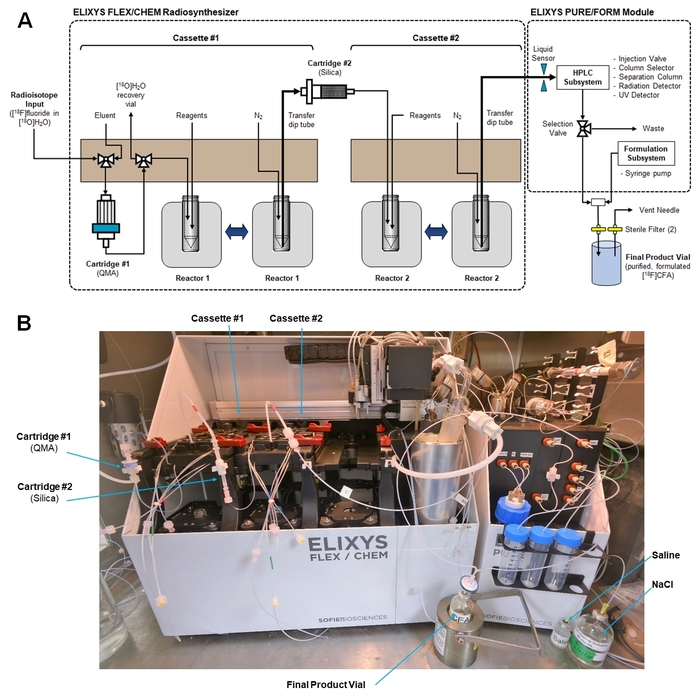
Figure 5: Radiosynthesizer set-up for the synthesis of [18F]CFA. (A) This is a schematic showing cassette fluid paths, connections to cartridges, and the connection to transfer final crude product from the radiosynthesis module to the purification/formulation module. (Both modules are controlled with a single computer and software interface.) (B) This is a photograph of the radiosynthesizer inside a hot cell after the preparation for [18F]CFA synthesis. Please click here to view a larger version of this figure.
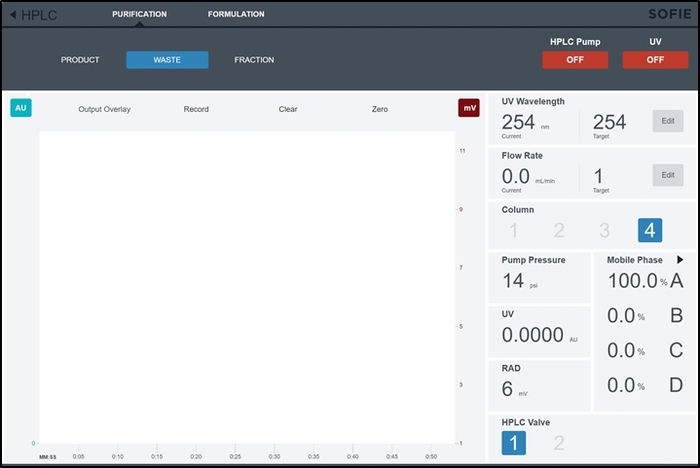
Figure 6: Screenshot of the purification/formulation module control interface. This screen is accessed by the operator to manually control the HPLC and formulation subsystems during the synthesis setup. Please click here to view a larger version of this figure.
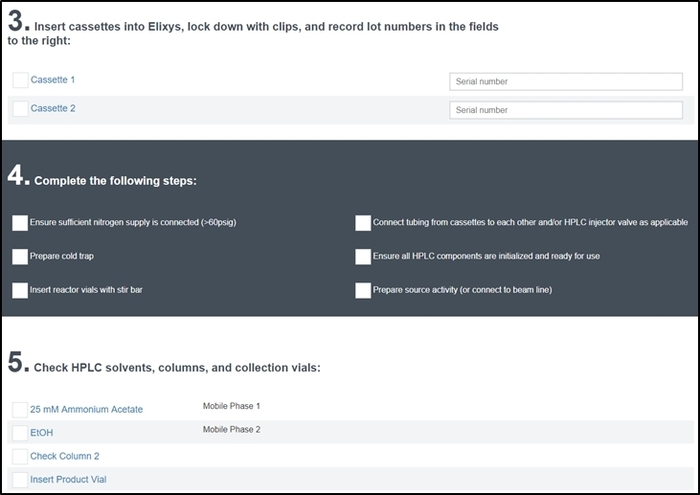
Figure 7: Pre-run checklist screen. The operator enters the serial number of the cassettes installed in the system and must check off each item to ensure the system has been properly configured and prepared for the synthesis. In addition to these sections, the operator is also prompted for a name and description of the synthesis run (Section 1) and lot numbers for all reagents used (Section 2) and is asked to verify all reactor video feeds are functioning correctly (Section 6). Please click here to view a larger version of this figure.
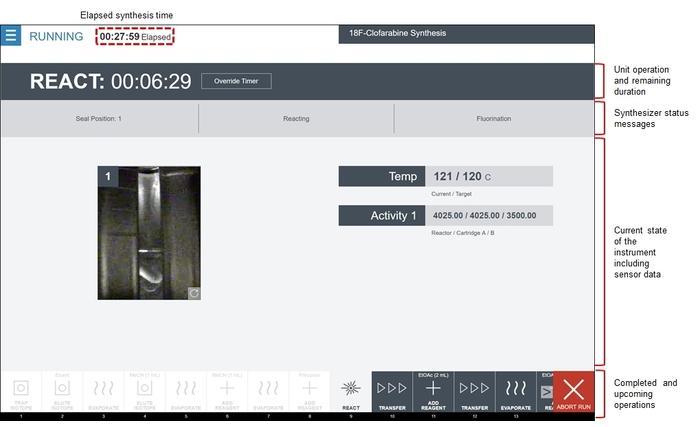
Figure 8: Screenshot of the radiosynthesizer software while running the [18F]CFA synthesis sequence. The software displays the order of unit operations in the filmstrip area. Completed operations are greyed out and highlighted in white, the current operation is highlighted in grey, and upcoming operations are shown in dark grey. The center area of the screen shows the status of the active unit operation, including which subcommand is being executed, as well as the current system status (reactor video feeds and sensor data). This particular React unit operation is the fluorination reaction. In the Temp area, the current temperature of the reactor is shown next to the target (programmed) temperature. Below this, the Activity area displays the radiation sensor values from the three sensors associated with the reaction step. Finally, a video feed on the left shows a live view of the reactor vial. Please click here to view a larger version of this figure.
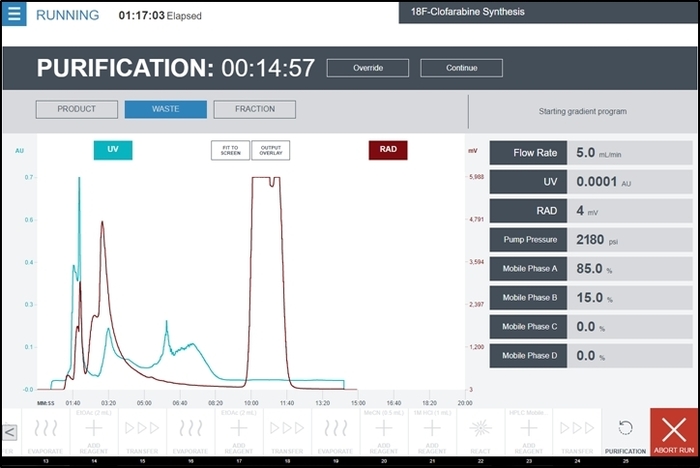
Figure 9: Screenshot of the radiosynthesizer user interface while running the Purification unit operation during the synthesis of [18F]CFA. The UV detector and radiation detector outputs of the purification/formulation module are displayed on the central graph in real time. Additional feedback from the detectors and HPLC pump are shown on the right side of the screen. The operator collects the product peak by temporarily selecting Product when the peak begins to appear and then switching back to Waste after the complete peak has been seen. Please click here to view a larger version of this figure.
Discussion
This protocol defines the basic steps that should be taken when automating a manual synthesis protocol to achieve the production of clinical grade tracer formulation. The entire development cycle, including quality control development, is exemplified by the radiotracer [18F]CFA (for the imaging of dCK activity). Particular attention was paid to modifying the automated synthesis to ensure the tracer's suitability for clinical use. The synthesis entails basic processes such as the activation of [18F]fluoride, radiofluorination of the precursor molecule, intermediate cartridge purification, protecting-group removal, and semi-preparative HPLC purification and formulation for injection. These basic processes comprise a standard repertoire that is sufficient for the synthesis of the vast majority of 18F-labeled PET tracers.
While designing the synthesis, the choice of reagents and their quality assurance is of particular importance for clinical use. Ensuring the correct programming and proper connections by performing a mock synthesis (solvents only) is imperative to eliminate unexpected errors when the synthesis is performed with radioactivity. The subsequent synthesis optimizations (solvents, volumes, amounts, temperatures, reaction times, and purification conditions) depend on the specific PET tracer in development. During these experiments, particular focus should be shone on the chemical and radiochemical purity of the final product that can be achieved, as these must meet stringent requirements for clinical use. A synthesis that reliably produces a pure product in lower but sufficient activity yields is usually preferred over a higher-yielding process that has a risk to fail sporadically. Once the synthesis has been adequately optimized, the final process needs to undergo validation tests (a regulatory requirement) to ensure clinical suitability. The validated synthesis method can then be used to produce the PET tracer for clinical use. When synthesizing a PET tracer according to a validated method, the standard operating procedures should be thoroughly followed. To ensure compliance, the software is programmed to have the operator confirm the completion of key steps via a pre-run checklist after clicking on Run to start the synthesis. While the system will perform the synthesis in an automated fashion, the purification step requires manual intervention. The operator must, therefore, closely observe the chromatographic screen during the HPLC purification step, and manually input in real-time when to start and stop collecting the product fraction.
Within our automation and optimization efforts for the [18F]CFA synthesis, we have streamlined the semi-preparative HPLC purification method of the product mixture by using an injectable solvent system consisting of ammonium acetate solution and EtOH; our previous method required an additional step to exchange the solvent after purification16. The subsequent formulation process, thus, needs only to reduce the EtOH content of the collected fraction to permitted levels, and to ensure its isotonicity, both of which can be accomplished by dilution. The formulation step was performed using a second program consisting of a single Formulation unit operation to allow variable volume additions of NaCl-solutions to the purified product fraction via the formulation module to account for the variable volume obtained after HPLC purification. If the collected product fraction volume was set to be constant instead, the Formulation unit operation could be included in the main synthesis program, avoiding the need for an independent program. An alternative approach to avoid manual intervention would be to use the full functionality of the formulation module (e.g., dilute the purified tracer with water, trap on a C18 solid-phase extraction cartridge, wash it, elute it with a fixed volume of EtOH, and finally, dilute it with a fixed volume of saline).
The technique presented here for automating and validating a synthesis protocol for clinical use is intended to be quite general. Through the choice of radiosynthesizer (ELIXYS), a wide range of syntheses can be automated and validated. This includes complex 3-pot syntheses, or syntheses involving high temperatures of volatile solvents. Optimizing a synthesis can be achieved by changing the parameters of the software program. The synthesizer has features to monitor the impact of changes, such as positioning the reaction vessels for the removal of samples for radio-TLC or radio-HPLC analysis. However, without system modifications, the system currently does not allow for the handling of very low reagent volumes (~5 - 20 µL), intermediate product distillation, or the handling of [18F]AlF, 68Ga, or other radiometals. If the manual synthesis to be automated contains such steps and they cannot be circumvented, automation and validation with another radiosynthesizer platform may be appropriate.
Although this work has focused on the development of a protocol for the automated production of [18F]CFA for clinical use, the synthesis of many other PET tracers could be automated in a manner suitable for clinical production, following the same logic and methods. Following the method presented here, we have also adapted the automated synthesis of 9-(4-[18F]fluoro-3-[hydroxymethyl]butyl)guanine ([18F]FHBG) and validated it for clinical use. User-established protocols can be uploaded to and downloaded from the SOFIE Probe Network, a web portal for sharing synthesis programs and associated documentation among different radiopharmacy sites27. This can avoid a duplication of efforts in the community and facilitate multi-center clinical studies involving PET imaging.
Disclosures
The Regents of the University of California have licensed technology to SOFIE that was invented by Jeffrey Collins and R. Michael van Dam and have taken equity in SOFIE as part of the licensing transaction. Furthermore, R. Michael van Dam is a founder and consultant of SOFIE. The terms of this arrangement have been reviewed and approved by the University of California, Los Angeles in accordance with its conflict of interest policies. Eric Schopf and Christopher Drake are employees and shareholders of SOFIE.
Acknowledgements
This work has been supported in part by the National Cancer Institute (R44 CA216539) and the UCLA Foundation from a donation made by Ralph and Marjorie Crump for the UCLA Crump Institute for Molecular Imaging.
Materials
| Name | Company | Catalog Number | Comments |
| ELIXYS FLEX/CHEM | Sofie (Culver City, CA, USA) | 1010001 | Radiosynthesizer |
| Radiosynthesizer cassette | Sofie (Culver City, CA, USA) | 1861030400 | Cassette for ELIXYS FLEX/CHEM |
| ELIXYS PURE/FORM | Sofie (Culver City, CA, USA) | 1510001 | Radiosynthesizer purification module |
| [O-18]H2O | IBA RadioPharma Solutions (Reston, VA, USA) | IBA.SP.065 | >90% isotopic purity |
| [F-18]fluoride in [O-18]H2O | UCLA | N/A | Produced in a cyclotron (RDS-112; Siemens; Knoxville, TN, USA) by the (p,n) reaction of [O-18]H2O. Bombardment at 11 MeV using a 1 mL tantalum target with havar foil. |
| Deionized water | UCLA | N/A | Purified to 18 MΩ and passed through 0.1 µm filter |
| Acetonitrile (MeCN) | Sigma-Aldrich (St. Louis, MO, USA) | 271004 | Anhydrous, 99.8% |
| Ethanol (EtOH) | Decon Laboratories, Inc. (King of Prussia, PA, USA) | 2701 | Anhydrous, 200 proof |
| Sodium hydroxide (NaOH) solution | Merck (Burlington, MA, USA) | 1.09137.1000 | 1M solution |
| Hydrochloric acid (HCl) solution | Fisher Chemical (Hampton, NH, USA) | SA48-500 | 1M solution |
| Ethyl acetate (EtAc) | Fisher Chemical (Hampton, NH, USA) | E195SK-4 | HPLC grade |
| Sodium chloride (NaCl) | Fisher Chemical (Hampton, NH, USA) | S-640-500 | USP grade |
| Ammonium acetate | Fisher Chemical (Hampton, NH, USA) | A639-500 | HPLC grade |
| Potassium carbonate (K2CO3) | Fisher Chemical (Hampton, NH, USA) | P-208-500 | Certified ACS |
| CFA precursor | CalChem Synthesis (San Diego, CA, USA) | N/A | Custom synthesis |
| Cryptand 222 (K222; Kryptofix 2.2.2) | ABX Advanced Biochemical Compounds (Radeberg, Germany) | 800.1000 | >99% |
| Sodium chloride (NaCl) solution (saline) | Hospira (Lake Forest, IL, USA) | 0409-4888-02 | 0.9%, for injection, USP grade |
| Silica cartridge | Waters (Milford, MA, USA) | WAT051900 | Sep-pak Classic |
| Quaternary methylammonium (QMA) cartridge | Waters (Milford, MA, USA) | WAT023525 | Sep-pak Light Plus |
| Sterile syringe filter (0.22 µm) | Millipore Sigma (Burlington, MA, USA) | SLGSV255F | Millex-GV |
| Glass V-vial (5 mL) | Wheaton (Millville, NJ) | W986259NG | Used for reaction vessels |
| Septa | Wheaton (Millville, NJ) | 224100-072 | Used for reagent vials |
| Crimp cap | Wheaton (Millville, NJ) | 224177-01 | Used for reagent vials |
| Amber serum vial (2 mL) | Voigt (Lawrence, KS, USA) | 62413P-2 | Used for reagent vials |
| Magnetic stir bar | Fisher Scientific (Hampton, NH, USA) | 14-513-65 | Used for reaction vessels |
References
- Phelps, M. E. Positron emission tomography provides molecular imaging of biological processes. Proceedings of the National Academy of Sciences. 97 (16), 9226-9233 (2000).
- Kitson, S., Cuccurullo, V., Ciarmiello, A., Salvo, D., Mansi, L. Clinical Applications of Positron Emission Tomography (PET) Imaging in Medicine: Oncology, Brain Diseases and Cardiology. Current Radiopharmaceuticalse. 2 (4), 224-253 (2009).
- Sengupta, D., Pratx, G. Imaging metabolic heterogeneity in cancer. Molecular Cancer. 15, 4(2016).
- Rabinovich, B. A., Radu, C. G. Imaging Adoptive Cell Transfer Based Cancer Immunotherapy. Current Pharmaceutical Biotechnology. 11 (6), 672-684 (2010).
- Matthews, P. M., Rabiner, E. A., Passchier, J., Gunn, R. N. Positron emission tomography molecular imaging for drug development. British Journal of Clinical Pharmacology. 73 (2), 175-186 (2012).
- Hargreaves, R. The Role of Molecular Imaging in Drug Discovery and Development. Clinical Pharmacology & Therapeutics. 83 (2), 349-353 (2008).
- Radiosynthesis Database of PET Probes (RaDaP). , Available from: http://www.nirs.qst.go.jp/research/division/mic/db2/ (2017).
- 18F-Database of Imaging Radiolabelled Compounds (DIRAC). , Centre National de la Recherche Scientifique. Available from: http://www.iphc.cnrs.fr/dirac/ (2013).
- Keng, P. Y., Esterby, M., van Dam, R. M. Emerging Technologies for Decentralized Production of PET Tracers. Positron Emission Tomography - Current Clinical and Research Aspects. Hsieh, C. -H. , InTechOpen. London, UK. 153-182 (2012).
- Lazari, M., Irribarren, J., Zhang, S., van Dam, R. M. Understanding temperatures and pressures during short radiochemical reactions. Applied Radiation and Isotopes. , 82-91 (2016).
- Lazari, M., et al. ELIXYS - a fully automated, three-reactor high-pressure radiosynthesizer for development and routine production of diverse PET tracers. European Journal of Nuclear Medicine and Molecular Imaging (EJNMMI) Research. 3 (1), 52(2013).
- Claggett, S. B., Quinn, K., Lazari, M., Esterby, J., Esterby, M., van Dam, R. M. A new paradigm for programming and controlling automated radiosynthesizer. Journal of Nuclear Medicine. 53 (suppl. 1), 1471-1471 (2012).
- Claggett, S. B., Quinn, K. M., Lazari, M., Moore, M. D., van Dam, R. M. Simplified programming and control of automated radiosynthesizers through unit operations. European Journal of Nuclear Medicine and Molecular Imaging (EJNMMI) Research. 3, 53(2013).
- Lazari, M., et al. Fully Automated Production of Diverse 18F-Labeled PET Tracers on the ELIXYS Multireactor Radiosynthesizer Without Hardware Modification. Journal of Nuclear Medicine Technology. 42 (3), 203-210 (2014).
- Lazari, M., et al. Fully-automated synthesis of 16β-18F-fluoro-5α-dihydrotestosterone (FDHT) on the ELIXYS radiosynthesizer. Applied Radiation and Isotopes. 103, 9-14 (2015).
- Collins, J., et al. Production of diverse PET probes with limited resources: 24 18F-labeled compounds prepared with a single radiosynthesizer. Proceedings of the National Academy of Sciences. 114 (43), 11309-11314 (2017).
- Drake, C., et al. Enzymatic Radiofluorination of Biomolecules: Development and Automation of Second Generation Prosthetic on ELIXYS Radiosynthesizer. Journal of Nuclear Medicine. 58 (supplement 1), 1(2017).
- Gobbi, L. C., et al. Identification of Three Novel Radiotracers for Imaging Aggregated Tau in Alzheimer's Disease with Positron Emission Tomography. Journal of Medicinal Chemistry. 60 (17), 7350-7370 (2017).
- Ippisch, R., Maraglia, B., Sutcliffe, J. Automated production of [18F]-F-Py-peptides. Journal of Nuclear Medicine. 57, 275(2016).
- Chen, H., et al. AMG 580: A Novel Small Molecule Phosphodiesterase 10A (PDE10A) Positron Emission Tomography Tracer. Journal of Pharmacology and Experimental Therapeutics. 352 (2), 327-337 (2015).
- Waldmann, C. M., et al. An Automated Multidose Synthesis of the Potentiometric PET Probe 4-[18F]Fluorobenzyl-Triphenylphosphonium ([18F]FBnTP). Molecular Imaging and Biology. 20 (2), 205-212 (2018).
- Ravert, H. T., et al. An improved synthesis of the radiolabeled prostate-specific membrane antigen inhibitor, [18F]DCFPyL. Journal of Labelled Compounds and Radiopharmaceuticals. 59 (11), 439-450 (2016).
- Betthauser, T. J., et al. Characterization of the radiosynthesis and purification of [18F]THK-5351, a PET ligand for neurofibrillary tau. Applied Radiation and Isotopes. 130, 230-237 (2017).
- Shu, C. J., et al. Novel PET probes specific for deoxycytidine kinase. Journal of Nuclear Medicine. 51 (7), 1092-1098 (2010).
- Kim, W., et al. [18F]CFA as a clinically translatable probe for PET imaging of deoxycytidine kinase activity. Proceedings of the National Academy of Sciences. 113 (15), 4027-4032 (2016).
- Barrio, M. J., et al. Human Biodistribution and Radiation Dosimetry of 18F-Clofarabine, a PET Probe Targeting the Deoxyribonucleoside Salvage Pathway. Journal of Nuclear Medicine. 58 (3), 374-378 (2017).
- SOFIE. Sofie Probe Network. , Available from: http://www.sofienetwork.com/ (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved