Method Article
Nest Building Behavior as an Early Indicator of Behavioral Deficits in Mice
In This Article
Summary
Here, we present a protocol to quantify nest building behavior in mice, which is known to be impaired in several neurological disorders and diseases. This protocol examines the utility of four materials and offers the opportunity to quantify the rater agreement in scoring, improving the validity and reliability of the assay.
Abstract
Nest building is an innate behavior in male and female rodents, even when raised in laboratory settings. As such, many researchers provide rodents synthetic and/or natural materials (such as twine, tissue, cotton, paper, and hay) as a gauge of their overall well-being and as an ancillary assessment to predict the possible decline in cognition. Typically, changes in nesting behaviors, such as failure to create a nest, indicate a change in health or welfare. In addition, nesting behavior is sensitive to many environmental and physiological challenges, as well as many genetic mutations underlying pathological disease states. The following protocol describes a nesting behavior paradigm that explores the usage of four types of nesting material. In addition, the protocol utilizes intraclass correlations to demonstrate that inter-rater reliability is higher when nests are constructed out of shredded paper compared to other common nesting materials such as cotton squares, paper twists, and soft cob bedding. The chosen methodology and statistical considerations (i.e., intraclass correlation) for this assay may be of interest for those conducting experiments assessing the quality of living of mice.
Introduction
Nest building is an innate behavior in many animals such as birds, fish, rodents, and great apes, and it is attracting more attention for its potential utility in the study of neurological diseases and disorders characterized by diminished well-being and impaired activities of daily living1. Mice, both male and female, build similarly-sized nests for reproductive purposes, heat conservation, and shelter; more importantly, they continue to do so even when raised in laboratory settings2. Hess, Petrovich, and Goodwin3 argue that biologically-appropriate stimuli are paramount to induce biologically-appropriate behaviors such as nesting; however, a variety of natural and artificial materials, such as hay, hemp, cotton twine, paper strips, cotton dental balls, and pressed cotton squares, have been used to assess nest building behavior2. Changes in these nesting behaviors (i.e., failure to create a nest out of the provided material) generally indicate a change in health or welfare. In most cases, a lack of nest building is attributed to several factors that negatively impact well-being. Such examples that have all effectively decreased the quality of nest building in mice include temperature extremes; painful stimulation; induced illness and infection; genetic mutations; and brain lesions in the medial preoptic areas, septum, and hippocampus4,5,6.
Alzheimer’s disease is a progressive neurodegenerative disease characterized by loss of brain tissue, accumulation of extracellular amyloid-β plaques, and intracellular neurofibrillary tangles composed hyperphosphorylated microtubule-associated protein, tau7. Additionally, Alzheimer’s disease is characterized by, most notably, deficits in learning and memory and impairments of activities of daily living. In mice, activities of daily living are commonly examined via circadian wheel-running8,9,10,11, although new alternatives, such as nesting, are growing in popularity. Nesting has been shown to be sensitive to manipulations (e.g., genetic mutations, environmental stressors) that have been identified as risk factors and/or causes of Alzheimer’s disease. As such, nesting can be used as an additional or complementary assay in many mouse strains that model those characteristics of Alzheimer’s disease. For example, Deacon and colleagues12 measured nest building of Tg2576 mice with the Swedish amyloid precursor protein (APPswe) and presenilin 1 (PS1) genetic mutations. The quality of nests constructed by group-housed Tg2576 mice was significantly worse than wildtype controls at both 3- and 12-months of age. In agreement with these findings, Filali et al.13 reported that singly housed APPswe/PS1 male mice given two pieces of 5 x 5 cm cotton built significantly poorer nests as rated on a scale of 1 - 5 (1 = nestlet not touched, 5 = a near perfect nest). Transgenic mice consistently built worse nests at 6-, 9-, and 12-months of age compared to their wildtype counterparts, and in some cases, the Appswe/PS1 mice completely failed to build a nest out of the provided cotton.
Previous research from our lab has demonstrated that wildtype C57BL/6J mice build significantly better nests out of cotton squares compared to CRND8 and CRND8/E4 mice9. However, the majority of experiments using pressed cotton squares appear to be variable, with wildtype mice failing to show expected high scores compared to transgenic mice that are expected to show very low scores in nesting2, which may in part lead to a lack of differences in estimated parameters (i.e., mean differences) and statistical significance. The lack of differences may be due in part to inadequate aging of mice or time allotted for nesting. Alternatively, nesting material may be an additional challenge, resulting in more variability due to researchers’ methodological preferences in the quantity and type of material, which may even interact with mouse strain. For example, Robinson-Junker and colleagues14 provided processed or unprocessed bedding material of different sizes (i.e., small or large flakes) to C3H/HeNCrl mice and BALB/cAnNCrl mice, which are commonly observed, respectively, as poor and strong nest builders. When provided unprocessed bedding, C3H mice built less complex, yet similar in quality nests compared to those of BALB/c mice. Likewise, Martin and colleagues15 compared nest complexity of different nesting materials given to deer mice, a distant relative of the Mus musculus species that have distinct evolutionary differences (i.e., more likely to burrow in trees and underbrush and are more active in captivity), but receive similar husbandry care as common laboratory mice and build nests out of any available soft, fibrous material16,17,18. Females and breeders with pups in the home cage built more complex nests than males, and the authors suggest that these behavioral differences may be due to associated changes in progesterone concentrations in deer mice15. More importantly, mice built more complex nests composed of brown paper followed by cotton squares and cotton cylinders, and the least complex nests were constructed out of white paper and dispersed mini-cotton squares.
Despite the growing popularity of nesting, considerations regarding scientifically valid, cost-effective, and time-sensitive practices are minimally discussed. Given the aforementioned methodological and economical challenges, this protocol investigates the utility of different nesting materials – cotton squares, paper twists, shredded paper, and processed bedding – in nesting behavior. Specifically, we provided all nesting materials to both aged C57BL/6J wildtype controls and Alzheimer’s disease APOE e4 mice in order to investigate any potential genotype by material interactions in nesting quality. Additionally, the experiment sought to assess inter-rater reliability of nests constructed out of different materials. Taken together, this protocol demonstrates the superiority of one nesting material in this sample – shredded paper – in terms of nest quality and scorer agreement, with the intention of improving the validity and reliability of the nesting assay.
Protocol
All procedures were approved by the George Mason University Institutional Animal Care and Use Committee and are in accordance with guidelines set forth by the Assessment and Accreditation of Laboratory Animal Care.
1. Animals and considerations prior to the assessment
- For this protocol, use adult, 9 - 12-month-old C57BL/6J (n = 10) (stock # 000664) wildtype mice and APOE e4 mice (n = 11) from a hemizygous J20 (stock #006293) x homozygous APOE e4 (stock #012307) cross.
- In the housing room, group-house mice with littermates of the same sex with appropriate enrichment (e.g., for this protocol, mice were provided a running wheel, igloo, and a small nylon chew toy). Group-house 4 - 6 female mice, and 4 males in a 356 mm L x 485 mm W x 218 mm H home cage.
NOTE: Researchers may consider implementing strategies prior to and/or after nesting in order to avoid cage-mate aggression when mice are reintroduced after nesting trials. Such strategies may include, but are not limited to, daily handling prior to behavioral testing to better acclimate mice to handling during behavioral testing, researchers, and husbandry staff19, separating and individually housing aggressive mice, or reducing the number of mice in the homecage20, depending on the severity of in-cage fighting, observed wounds21, etc.
2. Room and nesting set-up
- Ensure that each mouse completes four trials (1 material per trial). Randomize the order of nesting material for each mouse to avoid an order effect.
- Prepare the cages in a separate testing room. Record environmental conditions (e.g., 22.2 – 22.3°C, 45-47% humidity, lights on 9:00 AM – 9:00 PM) such that they are consistent across trials and are identical to the housing room. Provide food and water ad libitum.
- Assign each mouse a random identification (ID) number or letter. Attach the random identifier card to a 29.2 x 18.4 cm cage.
- Record the original animal ID/tag and other necessary identification in a colony record to ensure that assistants and animal husbandry staff remain blind to conditions.
- Randomly order cage placement in the testing room such that wildtype and transgenic mice are not inappropriately separated (i.e., on opposite sides of the room, on separate shelves, etc.).
- Prepare the nesting materials by sufficiently covering the bottom of the cage. Use approximately 100 g dry weight of corncob bedding for the square, twist, and shredded paper trials, and approximately 100 g of the soft cob bedding for the soft cob trial.
- If using a beaker to disperse the nesting material, then fill the beaker no more than 100 mL with corncob bedding or soft cob bedding.
- Place the first nesting material in the sequence prior to introducing the mouse into the cage. This protocol utilized (1) a single pressed cotton square, (2) a single paper twist, (3) 100g of soft cob bedding only (i.e., no additional bedding or nesting material added), and (4) 2.5 g of clean (no ink), shredded white printer paper cut into 5 – 7cm strips. Disperse nesting materials as shown in Figure 1 (baseline).

Figure 1: Cage set-up for each material. All mice completed one trial with each type of material for a total of four trials. Corncob bedding lined the entire bottom of the cages containing a paper twist, a pressed cotton square, and shredded paper. Soft cob bedding was evenly dispersed across the cage to encourage mice to separate out the small cotton squares from the corncob. Please click here to view a larger version of this figure.
3. Nesting trial
- Begin the first nesting trial at the same time of day at the start of the light cycle (e.g., 9:00 AM).
- Bring the homecage containing the mice into the testing room. Remove each mouse from the homecage and place it into its assigned nesting cage with the material already placed in the cage. Return the homecages to the housing room.
- Allow mice to complete 1 trial for 24 h undisturbed.
- 24 h after the start of the first trial, return to the testing room.
- Carefully remove the lid of the cage and photograph each mouse’s nest, capturing the assigned ID in the photograph and minimizing the appearance of any materials outside of the cage.
NOTE: Wait for the mouse to move off the nest before attempting to remove it from the cage. It is strongly recommended to photograph the nest while the mouse is in the cage but off the nest. Attempting to remove the mouse from the cage before photographing can potentially cause the mouse to become startled, thus move on top of, and disperse, nesting materials. - Gently remove the mouse from the nesting cage and place it in a temporary holding cage. Dispose of the bedding and any nesting materials, replace the bedding and provide the next nesting material in the sequence, and return the mouse to the nesting cage.
- Repeat as necessary to obtain scores for all 4 trials. It is recommended to use shredded paper and the accompanying scoring criteria, although researchers may be interested in using the alternative materials discussed in this protocol.
- When all trials are complete, return mice to their home cages. Observe the mice for any potential aggressive behavior. Aggression may occur in older wildtype males.
NOTE: For the purposes of this protocol, mice were tested once at approximately 9 – 12 months of age. Nesting should be conducted at several ages (i.e., at an earlier age, dependent on the chosen strain, prior to the onset of phenotypic traits) to document the decrease in nesting capacity over time and demonstrate the likely causative role of neurodegeneration.
4. Scoring and assessing inter-rater reliability
- Provide baseline images for each nesting material to at least 2 individuals blind to the study. Although not required, ensure that the scorers are familiar with the concept of nesting. When training scorers, provide a series of example nests (e.g., Figure 2 for shredded paper) to familiarize the scorers with each type of material (if applicable) and the scoring criteria.
- Evaluate each nest on a scale of 1 – 5 using the following scale information (adapted from Deacon, 2006)2. See Figure 2 as example scoring using shredded paper.
- Assign a score of 1 when the shredded paper or small squares remained scattered throughout the cage, or the cotton square or twist remained untouched;
- Assign a score of 2 when some of the material was constructed into a nest, but over 50% of the material was not used for nest construction (i.e., remained scattered or the majority of the original material remained untouched);
- Assign a score of 3 when a noticeable nest was constructed, but several pieces were still scattered;
- Assign a score of 4 when almost all the material was used for the nest, but few pieces of material remained scattered or were near the nest;
- Assign a score of 5 when all material was used to make an identifiable nest.
NOTE: Scorers should take breaks and revisit baseline photos in order to avoid fatigue and bias during the scoring procedure. Scorers should not discuss scores with one another to avoid bias. Scorers should discuss scores after scoring is complete, or if further discussion is needed to change scores, which can potentially resolve issues with intraclass correlations (ICC). Intraclass correlations will be conducted using the steps listed in section 4.2.
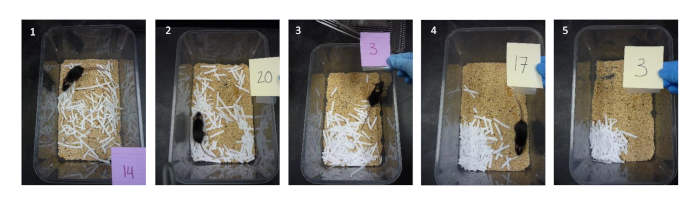
Figure 2: Example of scoring using the criteria for shredded paper, the preferred material. From left to right: 1 – the shredded paper remained untouched; 2 – some of the paper was constructed into a nest but most of the pieces remained scattered; 3 – a noticeable nest was constructed, but several pieces were still scattered; 4 – most pieces were used for the nest, but some pieces were scattered near the nest; 5 – all pieces were used to make the nest. Note that in the photographs, the animal’s assigned number is shown to avoid unblinding. The color of the card is random. Researchers should allow the mouse to remain in the cage in order to avoid startling the mouse, which could potentially disperse the nest. Please click here to view a larger version of this figure.
- Collect and input scores and format the data file such that each scorer’s scores for each material are in separate columns.
NOTE: If using Supplementary File 1, please note that the coding script for this analysis requires free, open source softwares (e.g., irr package in RStudio)22,23. The script removes headers and subjects variables (i.e., the top row and animal IDs) to conduct the intraclass correlation (ICC). Run the script to conduct a two-way, agreement, averages measures ICC by highlighting the demarcated section of the code and hitting Ctrl+Enter or Command+Enter. - Compare the ICC to existing criteria24,25,26. Typically, ICC values above 0.80 correspond to strong inter-rater reliability, justifying scores to be averaged at a given time point (Supplementary File 2).
- Use the same ICC criteria for intra-rater reliability, which may be of interest for those conducting scoring for the first time.
CAUTION: Scorers should score the same photographed nests within a week of the initial assessment. Proceed with caution with averaging data when ICC is low, as this may skew data or produce null findings. If ICC values are low, then (1) additional training for scorers may be required, (2) scorers may need to discuss their reasoning for nest scores, (3) another independent reviewer may be needed to make a judgment call regarding the scores, or (4) intra-rater reliability may need to be assessed. If intra-rater reliability is poor, then additional training or a different scorer may be required.
5. Statistical considerations
- Conduct statistical analyses, as appropriate. For data that do not violate normality assumptions but use multiple materials for each mouse (i.e., a within-subjects effect), use a mixed analysis of variance (ANOVA).
- If using codes provided as supplemental files, please download additional add-on packages which are specified in the script27,28,29,30.
- If conducting a mixed ANOVA using the provided script, run the script to convert any wide-format data files into long-format (i.e., instead of variables repeated across Columns, convert the cells to repeat across Rows). An example data file is included in this manuscript to demonstrate how to convert a data file from wide-format to long-format.
- Conduct the mixed ANOVA, as specified in the script. Note that if Mauchly’s test of Sphericity is violated, then implement correction factors such as Greenhouse-Geisser correction.
- Conduct any necessary posthoc tests to examine differences among factors with more than 2 levels, as specified in the coding script. In this example, conduct Bonferroni posthoc tests to compare the different types of nesting material.
NOTE: If only using one nesting material, then do not incorporate a within-subjects factor. If collecting data at multiple time points, however, incorporate these repeated-measures as if levels of a within-subjects factor.
NOTE: In some cases, wildtype mice may have perfect scores and thus will exhibit a ceiling effect, leading to a non-normal distribution2. Consider appropriate statistical tests (e.g., nonparametric tests with alternative measures of central tendency and dispersion), normalization methods, or robust approaches (mixed effects modeling for repeated measures) when analyzing data.
Results
Results from the four different nesting materials provided to wildtype and APOE e4 mice are explained as follows. Based on existing criteria, the ICC showed a strong agreement among all three coders for all four nesting materials (shredded paper ICC = 0.94; square ICC = 0.91; bedding ICC = 0.87; twist ICC = 0.87); therefore, the three scores were averaged together to create a single score for each material provided. A 2 x 4 mixed ANOVA yielded a significant main effect of genotype, F(1, 19) = 31.30, p < 0.01, ηp2 = 0.62. Across all four provided materials, wildtype mice scored higher on nest quality (3.18 ± 0.20) compared to APOE e4 mice (1.98 ± 0.16). In addition, the mixed ANOVA yielded a significant main effect of material, F(3, 57) = 57.48, p < 0.01, ηp2 = 0.75. Pairwise comparisons with Bonferroni correction showed that the shredded paper (4.11 ± 0.20) was rated significantly higher (p < 0.05) in quality than the square (1.95 ± 0.21), bedding (2.21 ± 0.21), or twist (1.94 ± 0.20) materials, with no differences seen between the square, bedding, and twist materials (all p > 0.99). There was no significant interaction between genotype and material. Data are shown in Figure 3.
Other experiments from our laboratory have yielded similar results in early-onset models of Alzheimer’s disease. For example, as demonstrated in Figure 4, 5.5-month-old P301L rTg4510 (htau) mice built significantly worse nests out of shredded paper compared to their age-matched wildtype counterparts31. Likewise, dual J20 (hAPP)/htau and single htau mice built poorer nests out of shredded paper compared to their wildtype and CAMKIIa-promoter only counterparts, both at 3.5- and 7-months of age32 (data not shown).
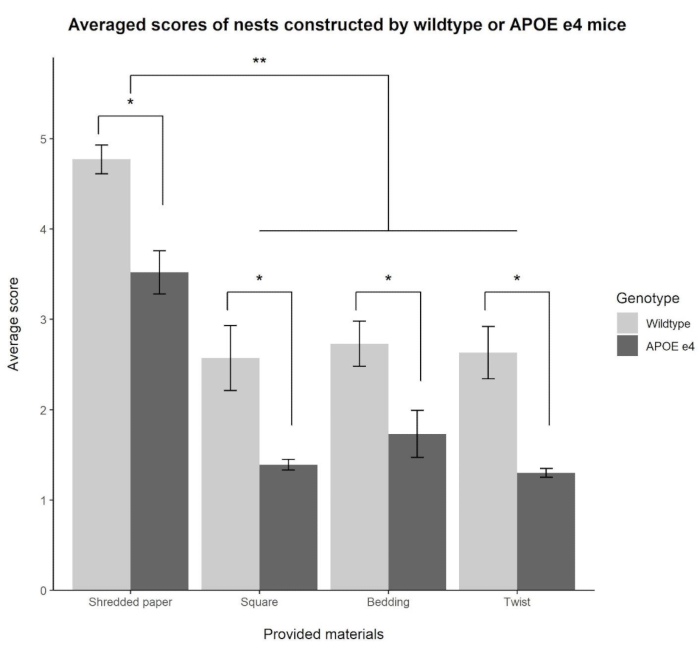
Figure 3: Rated quality of nests made from different materials provided to wildtype and APOE e4 mice. A main effect of genotype (*p < 0.01) demonstrated that wildtype mice consistently built better nests made from the shredded paper, pressed cotton squares, paper twist, and soft cob bedding compared to APOE e4 mice. The main effect of material (**p < 0.01) also demonstrated that nests constructed from the shredded paper were rated higher in quality compared to the three other materials. The shredded paper had the highest inter-rater reliability measure as assessed by ICC. Please click here to view a larger version of this figure.
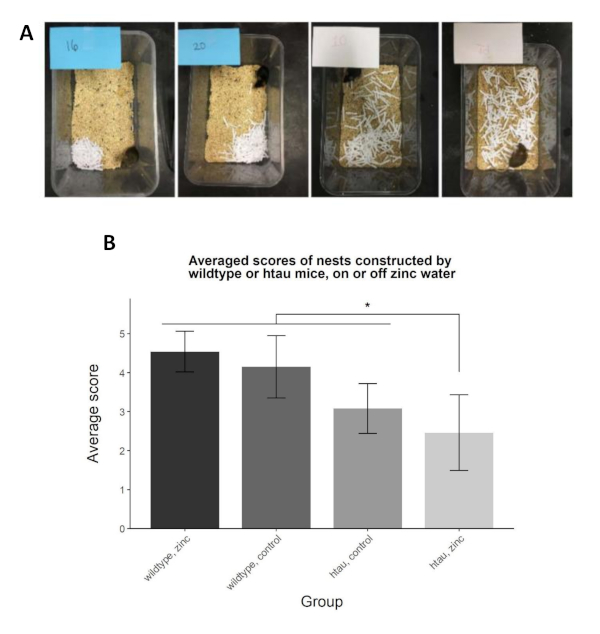
Figure 4: Shredded paper nests built by wildtype and htau mice, on or off zinc water. (A) Representative nests constructed by 5.5-month-old wildtype and htau mice, on control (tap) water or water supplemented with 10 parts per million zinc (a biometal implicated in Alzheimer’s disease). Nests were scored on the 1 - 5 scale using the specified criteria. From left to right: wildtype + zinc water (4.54±0.52); wildtype + control water (4.15±0.80); htau + control water (3.08 ± 0.64); htau + zinc water (2.46±0.97). (B) A genotype*water interaction demonstrated that htau mice on zinc water constructed significantly worse nests compared to the other groups (*); in addition, simple effects showed that htau mice on zinc water built worse nests than htau mice on control water. Wildtype mice on control water and zinc water-built nests of higher, and similar quality, compared to their htau counterparts. Reprinted from Journal of Alzheimer’s Disease, Vol 64, Craven, KM, Kochen WR, Hernandez CM, Flinn JM, Zinc Exacerbates Tau Pathology in a Tau Mouse Model, 671-630, Copyright (2018), with permission from IOS Press31. The publication is available at IOS Press through http://dx.doi.org/10.3233/JAD-180151. Please click here to view a larger version of this figure.
Supplementary File 1: Supplemental Coding File – nesting.R Please click here to download this file.

Supplementary File 2: AD Study nesting scores.csv Please click here to download this file.
Discussion
Nesting is an evolutionarily important rodent behavior and has been used to assess the activity of daily living and general well-being in mice2. The ease to conduct the test, its reliability, and its face validity make nesting a practical complement to many behavioral tests such as burrowing, circadian rhythm, and grooming. But, as nesting becomes more commonly utilized in the laboratory, the various combinations to conduct, quantify, and interpret nesting increase. As such, further research is needed to explore the best methodologically and practically sound procedures for nesting, such that the validity and reliability of the assay are not sacrificed for costs, time of testing, and other procedures that reduce testing burden.
Nest building quality is indeed sensitive to the type of bedding provided as well as genotype. Overall, wildtype mice built significantly better nests compared to APOE e4 mice regardless of the nesting material; however, both wildtype and APOE e4 mice build significantly higher quality nests out of shredded paper compared to the three other nesting materials. Other studies provide corroborating evidence regarding the shredded paper: mice constructed more complex nests out of shredded paper strips than with pressed cotton squares, tissue, and aspen bedding33. Furthermore, nests constructed out of shredded paper strips have been qualitatively evaluated as more "naturalistic" than those made of other materials, which are characterized by the shape of the nest itself and the height of the walls around the nest cavity in order to form a dome6,33. As such, selection of proper material for this assay is critical in order to better observe natural behavior in a relatively controlled environment, i.e., the laboratory setting. More importantly, although this protocol assessed nesting once at 9 – 12 months of age, we emphasize that nesting should be conducted at several ages. The simplicity of this protocol permits it to be conducted several times, ideally before the onset of deficits that accompany neurodegeneration. Repeated measurements afford the opportunity to document the likely causative role of neurodegeneration in decreased nesting ability.
Natural nest building has been shown to differ among mice of different background strains34, and as such, the overall quality and shape of the nest may differ not due to the transgene of interest, but due to the background strain. For example, the ancestors of C57BL/6 mice were considered "hole" nesters, whereas the ancestors of BALB/C mice were considered "surface" nesters35. Mice on the C3H background or crosses with this strain, such as the hybrid C3H/He-C57BL/6 with E4 used in Graybeal et al.9 are also considered to be poor nesters; thus, researchers should strongly consider using control mice on the same background as transgenic mice, which would overall improve the direct, causative role of the transgene(s), rather than the background, in neurodegeneration and subsequent deficits in nest building behavior.
Some experiments often utilize a single scorer to qualitatively judge the complexity of the nest; however, we make the argument to include more scorers, and more importantly, scorers blind to experimental conditions. Through this approach, we utilized three independent, blind scorers and an intraclass correlation to assess the agreement among the scorers, who, with basic training, yielded high intraclass correlations which were indicative of high inter-rater reliability and strong agreement regarding nest quality. Furthermore, scores of nests composed of shredded paper and cotton squares had the highest intraclass correlations, an indication of stronger agreement and greater consistency among the scorers. The strong agreement also provides justification to average the multiple scores together, a strategy haphazardly implemented in behavioral research. Although this strategy requires more individuals and, presumably, more time to score nests, it effectively reduces bias in qualitative assessments such as nesting.
The nesting materials used in this assay were approximately equal in price except for the soft cob bedding. Commercial bedding for nesting may be economically resourceful for some experimenters; however, Martin et al.15 note that cotton squares, when compared to other materials such as crinkled paper, is one of the most expensive materials available for purchase. This may be due to easy availability, storage, and administration, but other researchers may opt for similarly valid and reliable materials, especially when challenged by large numbers of animals in a facility, the number of nesting trials, time restrictions for scoring nests, and high cage costs. Thus, shredded paper may be a more feasible and appropriate option. In addition, data capture for our method can be conducted immediately (i.e., scoring in person), although photographs should be highly considered in order to record, save, and re-quantify nests, if desired for the purposes of assessing inter- and intra-rater reliability at later times. As noted, we strongly emphasize the inclusion of multiple scorers for "test, re-test" practices to assess for agreement, as these methodologically sound procedures are often overlooked.
In conclusion, we believe that the methodology and statistical considerations (i.e., intraclass correlation) for this assay may be of interest for experiments assessing the quality of living, general well-being, and activities of daily living in mice.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Kevin Quant, Mario Martinez, and Edwin Portillo for scoring nests, Rachel Barkey for assisting in the preparation for filming, and Stephen Lippi and Deborah Neely for proofreading this manuscript. We would also like to thank the Department of Psychology for covering the costs of cages for this experiment, and the Krasnow Institute Animal Facility caretakers for their support.
Materials
| Name | Company | Catalog Number | Comments |
| Corncob bedding | Envigo | 7092 | 1/8 in bedding for cotton squares, shredded paper, and paper twist trials |
| Cotton Squares | Envigo | Iso-Blox | |
| Diamond Twists | Envigo | 7979C.CS | Paper twists used in protocol |
| Mouse - APOE4 e4 | Jackson Laboratories | #012307 | Homozygous APEO4 e4 mouse bred with hemizyous J20 mouse |
| Mouse - C57BL/6J | Jackson Laboratories | #000664 | Wildtype mouse for controls |
| Mouse - J20 | Jackson Laboratories | #006293 | Hemizygous mouse bred with the homozygous APOE e4 mouse to generate cross |
| Rstudio | R Core Team | V1.1.463 | Run with R version 3.5.3 (2019-03-11) -- "Great Truth". Note: additional R Packages are included in provided code and can be installed from CRAN |
| Soft Cob | Envigo | 7087C |
References
- Torres-Lista, V., Gimenez-Llorta, L. Impairment of nesting behavior in 3cTg-AD mice. Behavioural Brain Research. 247, 153-157 (2013).
- Deacon, R. Assessing nest building in mice. Nature Protocols. 1 (3), 1117-1119 (2006).
- Hess, E., Petrovich, S., Goodwin, E. Induction of Parental Behavior in Japanese Quail. Journal of Comparative and Physiological Psychology. 90 (3), 244-251 (1976).
- Deacon, R. Hippocampal cytotoxic lesion effects on species-typical behaviors in mice. Behavioural Brain Research. 132, 203-213 (2002).
- Deacon, R. Effects of medial prefrontal cortex cytotoxic lesions in mice. Behavioural Brain Research. 139, 139-155 (2003).
- Gaskill, B. N., Karas, A. Z., Garner, J. P., Pritchett-Corning, K. R. Nest building as an indicator of health and welfare in laboratory mice. Journal of Visualized Experiments. (82), 51012 (2013).
- Mayeux, R., Stern, Y. Epidemiology of Alzheimer Disease. Cold Spring Harbor Perspectives in Medicine. 2 (8), 006239 (2012).
- Boggs, K. N., Kakalec, P. A., Smith, M. L., Howell, S. N., Flinn, J. M. Circadian wheel running behavior is altered in an APP/E4 mouse model of late onset Alzheimer's disease. Physiology and Behavior. 182, 137-142 (2017).
- Graybeal, J. J., et al. Human ApoE ε4 alters circadian rhythm activity, IL-1β, and GFAP in CRND8 mice. Journal of Alzheimer's Disease. 43 (3), 823-834 (2015).
- Leng, Y., Musiek, E. S., Hu, K., Cappuccio, F. P., Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. The Lancet Neurology. 18 (3), 307-318 (2019).
- Zhou, L., Gao, Q., Nie, M., Gu, J. L., Hao, W., Wang, L., Cao, J. M. Degeneration and energy shortage in the suprachiasmatic nucleus underlie the circadian rhythm disturbance in ApoE-/- mice: Implications for Alzheimer's disease. Scientific Reports. 6, 36335 (2016).
- Deacon, R., et al. Age-dependent and -independent behavioral deficits in Tg2576 mice. Behavioural Brain Research. 189, 126-138 (2008).
- Filali, M., Lalonde, R., Riverst, S. Cognitive and non-cognitive behaviors in an APPswe/PS1 begenic model of Alzheimer's disease. Genes, Brain and Behavior. 8, 143-148 (2009).
- Robinson-Junker, A., Morin, A., Protchett-Corning, K., Gaskill, B. Sorting it out: Bedding particle size and nesting material processing method affects nest complexity. Laboratory Animals. 51 (2), 170-180 (2017).
- Martin, T., Balser, S., Young, G., Lewis, S. Cost and effectiveness of commercially available nesting substrates for Deer Mice (Peromyscus maniculatus). Journal of the American Association for Laboratory Animal Science. 55 (4), 412-418 (2016).
- Crossland, J. P., et al. Caring for Peromyscus spp. in research environments. Lab Animal. 43 (5), 162-166 (2014).
- Eisenberg, J. Studies on the Behavior of Peromyscus maniculatus gambelii and Peromyscus californicus parasiticus. Behaviour. 19 (3), 177-207 (1962).
- Joyner, C. P., Myrick, L. C., Crossland, J. P., Dawson, W. D. Deer mice as laboratory animals. ILAR Journal. 39 (4), 322-330 (1998).
- Neely, C., Lane, C., Torres, J., Flinn, J. The Effect of gentle handling on depressive-like behavior in adult male mice: Considerations for human and rodent interactions in the laboratory. Behavioural Neurology. , 2976014 (2018).
- Van Loo, P. L., Mol, J. A., Koolhaas, J. M., Van Zutphen, B. F., Baumans, V. Modulation of aggression in male mice: Influence of group size and cage size. Physiology & Behavior. 72 (5), 675-683 (2001).
- Kappel, S., Hawkins, P., Mendl, M. T. To group or not to group? Good practice for housing male laboratory mice. Animals. 7 (12), 88 (2017).
- Cicchetti, D. V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment. 6 (4), 284-290 (1994).
- Hallgren, K. A. Computing inter-rater reliability for observational data: An overview and tutorial. Tutorials in Quantitative Methods for Psychology. 8 (1), 23-34 (2012).
- McGraw, K. O., Wong, S. P. Forming inferences about some intraclass correlation coefficients. Psychological Methods. 1 (1), 30-46 (1996).
- . ggsignif: Significance Brackets for 'ggplot2'. R package version 0.5.0 Available from: https://CRAN.R-project.org/package=ggsignif (2019)
- . ez: Easy Analysis and Visualization of Factorial Experiments. R package version 4.4-0 Available from: https://CRAN.R-project.org/package=ez (2016)
- Lenth, R. V. Least-Squares Means: The R Package lsmeans. Journal of Statistical Software. 69 (1), 1-33 (2016).
- Wickham, H. . Ggplot2: Elegant Graphics for Data Analysis. , (2016).
- Craven, K. M., Kochen, W. R., Hernandez, C. M., Flinn, J. M. Zinc exacerbates tau pathology in a tau mouse model. Journal of Alzheimer's Disease. 64, 617-630 (2018).
- Lippi, S. L., Smith, M. L., Flinn, J. M. A novel hAPP/htau mouse model of Alzheimer's Disease: Inclusion of APP with tau exacerbates behavioral deficits and zinc administration heightens tangle pathology. Frontiers in Aging Neuroscience. 10, 382 (2018).
- Hess, S. E., et al. Home improvement: C57BL/6J mice given more naturalistic nesting materials build better nests. Journal of the American Association for Laboratory Animal Science. 47 (6), 25-31 (2008).
- Jirkof, P. Burrowing and nest building behavior as indicators of well-being in mice. Journal of Neuroscience Methods. 234, 139-146 (2014).
- Van Oortmerssen, G. Biological significance, genetics and evolutionary origin of variability in behaviour within and between strains of mice (Mus musculus). A behavior genetic study. Behaviour. 38 (1), 1-92 (1971).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved